- Record: found
- Abstract: found
- Article: found
ALKBH5‐mediated m6A modification of lncRNA KCNQ1OT1 triggers the development of LSCC via upregulation of HOXA9
Read this article at
Abstract
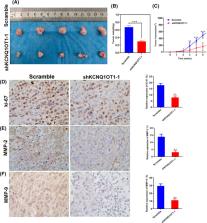
It has been shown that N6‐methyladenosine (m6A) modification is involved in the development of complex human diseases, especially in the development of cancer. Our research investigated the role and mechanism of the m6A modification of lncRNA KCNQ1 overlapping transcript 1 (KCNQ1OT1) in Laryngeal squamous cell carcinoma (LSCC) progression. Microarray analysis was used to quantitatively detect the m6A apparent transcriptional modification level of lncRNA in LSCC tissue. Methylated RNA immunoprecipitation‐qPCR (MeRIP‐qPCR), in situ hybridization (ISH) and quantitative real‐time PCR (qRT‐PCR) were used to examine the m6A modification and expression of KCNQ1OT1. In addition, in vivo and in vitro experiments have tested the effects of KCNQ1OT1 knockdown on the proliferation, invasion and metastasis of LSCC. Mechanically, we found the N6‐methyladenosine (m6A) demethylase ALKBH5 mediates KCNQ1OT1 expression via an m6A‐YTHDF2‐dependent manner and KCNQ1OT1 could directly bind to HOXA9 to further regulate the proliferation, invasion and metastasis of LSCC cells. In general, our research indicates that ALKBH5‐mediated m6A modification of KCNQ1OT1 triggers the development of LSCC via upregulation of HOXA9.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics, 2012.
- Record: found
- Abstract: found
- Article: not found
m6A-dependent regulation of messenger RNA stability
- Record: found
- Abstract: found
- Article: not found