- Record: found
- Abstract: found
- Article: found
Efficacy of a mobile application for smoking cessation in young people: study protocol for a clustered, randomized trial
Read this article at
Abstract
Background
Tobacco consumption is the most preventable cause of morbidity-mortality in the world. One aspect of smoking cessation that merits in-depth study is the use of an application designed for smartphones (app), as a supportive element that could assist younger smokers in their efforts to quit. To assess the efficacy of an intervention that includes the assistance of a smoking cessation smartphone application targeted to young people aged 18 to 30 years who are motivated to stop smoking.
Methods/design
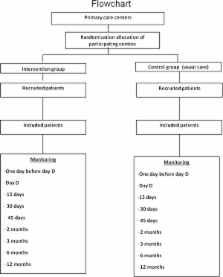
Cluster randomised clinical trial. Setting: Primary Health Care centres (PHCCs) in Catalonia. Analyses based on intention to treat. Participants: motivated smokers of 10 or more cigarettes per day, aged 18 to 30 years, consulting PHCCs for any reason and who provide written informed consent to participate in the trial. Intervention group will receive a 6-month smoking cessation programme that implements recommendations of a Clinical Practice Guideline, complemented with a smartphone app designed specifically for this programme. Control group will receive the usual care. The outcome measure will be abstinence at 12 months confirmed by exhaled-air carbon monoxide concentration of at least 10 parts per million at each control test.
Discussion
To our knowledge this is the first randomised controlled trial of a programme comparing the efficacy of usual care with a smoking cessation intervention involving a mobile app. If effective, the modality could offer a universal public health management approach to this common health concern.
Related collections
Most cited references5
- Record: found
- Abstract: found
- Article: found
Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011
- Record: found
- Abstract: found
- Article: not found
Txt2stop: a pilot randomised controlled trial of mobile phone-based smoking cessation support.
- Record: found
- Abstract: found
- Article: not found