- Record: found
- Abstract: found
- Article: not found
The role of berberine in Covid-19: potential adjunct therapy
Read this article at
Abstract
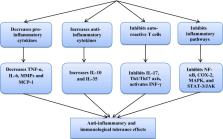
Coronavirus disease 2019 (Covid-19) is a global diastrophic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Covid-19 leads to inflammatory, immunological, and oxidative changes, by which SARS-CoV-2 leads to endothelial dysfunction (ED), acute lung injury (ALI), acute respiratory distress syndrome (ARDS), and multi-organ failure (MOF). Despite evidence illustrating that some drugs and vaccines effectively manage and prevent Covid-19, complementary herbal medicines are urgently needed to control this pandemic disease. One of the most used herbal medicines is berberine (BBR), which has anti-inflammatory, antioxidant, antiviral, and immune-regulatory effects; thus, BBR may be a prospective candidate against SARS-CoV-2 infection. This review found that BBR has anti-SARS-CoV-2 effects with mitigation of associated inflammatory changes. BBR also reduces the risk of ALI/ARDS in Covid-19 patients by inhibiting the release of pro-inflammatory cytokines and inflammatory signaling pathways. In conclusion, BBR has potent anti-inflammatory, antioxidant, and antiviral effects. Therefore, it can be utilized as a possible anti-SARS-CoV-2 agent. BBR inhibits the proliferation of SARS-CoV-2 and attenuates the associated inflammatory disorders linked by the activation of inflammatory signaling pathways. Indeed, BBR can alleviate ALI/ARDS in patients with severe Covid-19. In this sense, clinical trials and prospective studies are suggested to illustrate the potential role of BBR in treating Covid-19.
Related collections
Most cited references122

- Record: found
- Abstract: found
- Article: found
NF-κB signaling in inflammation
- Record: found
- Abstract: found
- Article: not found
Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods
- Record: found
- Abstract: found
- Article: not found
