- Record: found
- Abstract: found
- Article: found
Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride

Read this article at
Abstract
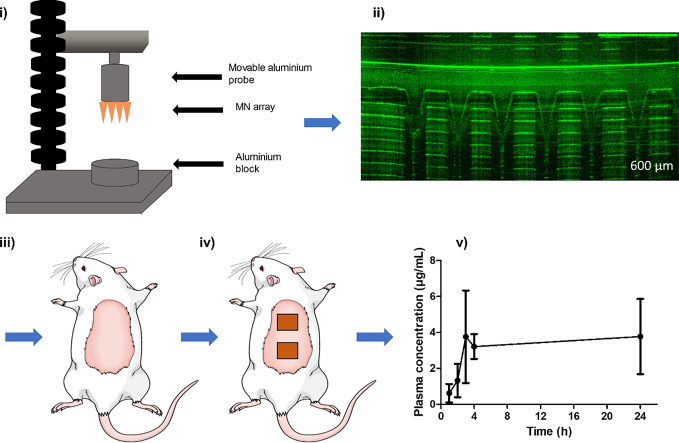
We investigated, for the first time, the potential for a hydrogel-forming microneedle (MN) patch to deliver the high-dose drug metformin HCl transdermally in a sustained manner. This may minimize some gastrointestinal side effects and small intestine absorption variations associated with oral delivery. Patches (two layers) were assembled from a lyophilised drug reservoir layer, with the MN layer made from aqueous blend of 20% w/w poly (methylvinylether- co-maleic acid) crosslinked by esterification with 7.5% w/w poly (ethylene glycol) 10,000 Da. >90% of metformin was recovered from homogeneous drug reservoirs. Drug reservoir dissolution time in PBS (pH 7.4) was <10 min. MN penetrated a validated skin model Parafilm® M consistently. Permeation of metformin HCl across dermatomed neonatal porcine skin in vitro was enhanced by using MN. The combined MN and metformin HCl reservoir patch (containing 75 mg or 50 mg metformin HCl, respectively) delivered 9.71 ± 2.22 mg and 10.04 ± 1.92 mg at 6 h, respectively, and 28.15 ± 2.37 mg and 23.25 ± 3.58 mg at 24 h, respectively.In comparison, 0.34 ± 0.39 mg and 0.85 ± 0.68 mg was delivered at 6 h, respectively, and 0.39 ± 0.39 mg and 1.01 ± 0.84 mg was delivered at 24 h, respectively, from a control set-up employing only the drug reservoirs. In vivo, metformin HCl was detected in rat plasma at 1 h post MN application at a concentration of 0.62 ± 0.51 μg/mL, increasing to 3.76 ± 2.58 μg/ml at 3 h. A maximal concentration of 3.77 ± 2.09 μg/ml was achieved at 24 h. C ss was 3.2 μg/mL. Metformin transdermal bioavailability using MNs was estimated as 30%.Hydrogel-forming MN are a promising technology that has demonstrated successful transdermal delivery of metformin HCl. Potential clearly exists for administration of other high-dose drugs using this system.
Graphical abstract
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery
- Record: found
- Abstract: found
- Article: not found
Accumulation of metformin by tissues of the normal and diabetic mouse.
- Record: found
- Abstract: not found
- Article: not found