- Record: found
- Abstract: found
- Article: found
Surveillance to Track Progress Toward Polio Eradication — Worldwide, 2017–2018
research-article

Jaymin C. Patel , PhD
1
,
,
Ousmane M. Diop , PhD
2 ,
Tracie Gardner , PhD
2 ,
Smita Chavan , MS
1 ,
Jaume Jorba , PhD
3 ,
Steven G. F. Wassilak , MD
1 ,
Jamal Ahmed , MD
2 ,
Cynthia J. Snider , PhD
1
05 April 2019
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
When the Global Polio Eradication Initiative (GPEI) began in 1988, cases of poliomyelitis
were reported from 125 countries. Since then, only Afghanistan, Nigeria, and Pakistan
have experienced uninterrupted transmission of wild poliovirus (WPV). The primary
means of detecting poliovirus is through surveillance for acute flaccid paralysis
(AFP) among children aged <15 years with testing of stool specimens for WPV and vaccine-derived
polioviruses (VDPVs) in World Health Organization (WHO)–accredited laboratories of
the Global Polio Laboratory Network (GPLN) (
1
,
2
). AFP surveillance is supplemented by environmental surveillance for polioviruses
in sewage at selected locations. Analysis of genomic sequences of isolated polioviruses
enables assessment of transmission by time and place, potential gaps in surveillance,
and emergence of VDPVs (
3
). This report presents 2017–2018 poliovirus surveillance data, focusing on 31 countries*
identified as high-priority countries because of a “high risk of poliovirus transmission
and limited capacity to adequately address those risks” (
4
). Some of these countries are located within WHO regions with endemic polio, and
others are in regions that are polio-free. In 2018, 26 (84%) of the 31 countries met
AFP surveillance indicators nationally; however, subnational variation in surveillance
performance was substantial. Surveillance systems need continued strengthening through
monitoring, supervision, and improvements in specimen collection and transport to
provide sufficient evidence for interruption of poliovirus circulation.
Acute Flaccid Paralysis Surveillance
Two surveillance performance indicators assess the quality of AFP surveillance. The
first is the nonpolio AFP (NPAFP) rate (the number of NPAFP cases per 100,000 children
aged <15 years per year); an NPAFP rate ≥2 is considered sufficiently sensitive to
detect circulating poliovirus. The second indicator is the collection of adequate
stool specimens (i.e., two stool specimens collected ≥24 hours apart and within 14
days of paralysis onset) and arrival at a WHO-accredited laboratory by reverse cold
chain and in good condition (i.e., without leakage or desiccation) from ≥80% of persons
with AFP, which ensures sensitivity and provides the specificity to track poliovirus
circulation (
2
).
Among the 47 countries in the WHO African Region (AFR), the NPAFP rate in 2017 was
7.0 per 100,000 children aged <15 years, and 92% of AFP cases had adequate stool specimens;
in 2018, the NPAFP rate was 5.4 per 100,000 children aged <15 years, and 89% of the
AFP cases had adequate stool specimens. Among the 18 high-priority AFR countries assessed,
15 (83%) met both surveillance indicators nationally in 2018, compared with 13 (72%)
in 2017 (Table 1). However, national indicators obscure subnational underperformance
(Figure). During 2017–2018, no WPV cases were reported in AFR; however, circulating
VDPV type 2 (cVDPV2) cases were reported in four countries. In 2017, the Democratic
Republic of the Congo accounted for all 22 reported cVDPV2 cases in AFR; in 2018,
65 cVDPV2 cases were reported in the region, including 20 in the Democratic Republic
of the Congo, one in Mozambique, 10 in Niger, and 34 in Nigeria (Table 1).
TABLE 1
National and subnational acute flaccid paralysis (AFP) performance surveillance indicators
and number of confirmed wild poliovirus (WPV) and circulating vaccine-derived poliovirus
(cVDPV) cases, by country — 31 Global Polio Eradication Initiative 2018–2020 high-priority
countries, World Health Organization (WHO) African, Eastern Mediterranean, South-East
Asia, and Western Pacific regions, 2017–2018*
WHO region/Country/Year
No. of AFP cases (all ages)
Regional/National NPAFP rate†
Subnational areas with NPAFP rate ≥2 (%)§
Regional or national AFP cases with adequate specimens (%)¶
Subnational areas with ≥80% adequate specimens (%)
Population living in areas meeting both indicators (%)**
No. of confirmed WPV cases*
No. of confirmed cVDPV cases*,††
2017
African Region
31,538
7
N/A
92
N/A
N/A
—¶¶
22
Burkina Faso
309
3.6
92
85
77
58
—¶¶
—¶¶
Burundi
145
2.8
53
83
65
11
—¶¶
—¶¶
Cameroon
970
9.0
100
86
90
75
—¶¶
—¶¶
Central African Republic
167
8.0
100
80
43
0
—¶¶
—¶¶
Chad
703
10.0
100
79
52
56
—¶¶
—¶¶
Democratic Republic of the Congo
2,148
5.1
100
79
42
32
—¶¶
22
Equatorial Guinea
12
2.5
57
17
14
0
—¶¶
—¶¶
Ethiopia
1,096
2.6
73
86
100
49
—¶¶
—¶¶
Guinea
452
8.4
100
88
100
86
—¶¶
—¶¶
Guinea Bissau
83
10.6
100
82
67
35
—¶¶
—¶¶
Kenya
479
2.3
66
83
68
36
—¶¶
—¶¶
Liberia
81
4.0
100
81
60
63
—¶¶
—¶¶
Mali
259
2.9
100
86
89
91
—¶¶
—¶¶
Mozambique
385
2.8
82
85
55
39
—¶¶
—¶¶
Niger
682
6.2
100
70
0
0
—¶¶
—¶¶
Nigeria
16,468
19.6
100
98
100
100
—¶¶
—¶¶
Sierra Leone
78
2.5
100
77
75
57
—¶¶
—¶¶
South Sudan
388
7.3
90
84
60
67
—¶¶
—¶¶
Eastern Mediterranean Region
19,192
8.4
N/A
88
N/A
N/A
22
74
Afghanistan
3,094
20.0
100
94
100
97
14
—¶¶
Djibouti
4
1.3
17
100
17
0
—¶¶
—¶¶
Iraq
699
4.5
95
87
79
74
—¶¶
—¶¶
Jordan
116
3.3
100
100
100
100
—¶¶
—¶¶
Lebanon
75
5.3
100
80
83
90
—¶¶
—¶¶
Libya
88
4.9
100
97
100
100
—¶¶
—¶¶
Pakistan
10,330
15.0
100
85
100
99
8
—¶¶
Somalia
345
5.0
100
99
100
100
—¶¶
—¶¶
Sudan
570
3.5
100
96
100
100
—¶¶
—¶¶
Syria
364
4.3
79
76
50
38
—¶¶
74
Yemen
713
6.3
100
82
70
68
—¶¶
—
South-East Asia Region
43,390
8.1
N/A
86
N/A
N/A
—¶¶
—
Indonesia
1,740
2.4
71
82
47
22
—¶¶
—
Western Pacific Region
6,634
2.0
N/A
90
N/A
N/A
—¶¶
—
Papua New Guinea
28
0.9
10
46
15
0
—¶¶
—
2018
African Region
24,849
5.4
N/A
89
N/A
N/A
—¶¶
65
Burkina Faso
357
4.0
100
86
77
58
—¶¶
—¶¶
Burundi
123
2.4
53
89
71
11
—¶¶
—¶¶
Cameroon
778
7.2
100
83
80
73
—¶¶
—¶¶
Central African Republic
133
6.5
86
68
14
0
—¶¶
—¶¶
Chad
650
9.0
96
90
78
56
—¶¶
—¶¶
Democratic Republic of the Congo
2,742
6.6
96
78
58
29
—¶¶
20
Equatorial Guinea
30
6.2
86
93
71
0
—¶¶
—¶¶
Ethiopia
1,083
2.5
73
83
55
49
—¶¶
—¶¶
Guinea
232
4.2
100
89
88
81
—¶¶
—¶¶
Guinea Bissau
96
12.0
100
78
44
35
—¶¶
—¶¶
Kenya
644
3.1
85
87
74
36
—¶¶
—¶¶
Liberia
72
3.6
100
85
67
43
—¶¶
—¶¶
Mali
292
3.2
100
87
78
91
—¶¶
—¶¶
Mozambique
463
3.4
91
87
73
39
—¶¶
1
Niger
973
8.5
100
81
75
0
—¶¶
10
Nigeria
9,400
10.9
100
95
100
100
—¶¶
34
Sierra Leone
114
3.5
100
83
75
57
—¶¶
—¶¶
South Sudan
430
8.0
100
83
60
67
—¶¶
—¶¶
Eastern Mediterranean Region
21,834
9.5
N/A
90
N/A
N/A
33
12
Afghanistan
3,376
21.6
100
94
97
98
21
—¶¶
Djibouti
0
0
N/A
N/A
N/A
N/A
—¶¶
—¶¶
Iraq
1,023
6.5
100
90
95
78
—¶¶
—¶¶
Jordan
115
3.3
100
100
100
100
—¶¶
—¶¶
Lebanon
89
6.5
100
97
100
94
—¶¶
—¶¶
Libya
122
6.8
100
96
100
100
—¶¶
—¶¶
Pakistan
12,190
17.5
100
87
88
99
12
—¶¶
Somalia
354
4.9
100
98
100
100
—¶¶
12
Sudan
579
3.4
100
97
100
100
—¶¶
—¶¶
Syria
362
5.5
93
85
86
44
—¶¶
—¶¶
Yemen
730
6.4
100
92
100
66
—¶¶
—¶¶
South-East Asia Region
40,493
7.6
N/A
85
N/A
N/A
—¶¶
1
Indonesia
1,636
2.3
62
82
44
22
—¶¶
1
Western Pacific Region
6,828
2.0
N/A
88
N/A
N/A
—¶¶
26
Papua New Guinea
282
8.1
82
46
18
0
—¶¶
26
Abbreviations: N/A = not applicable; NPAFP = nonpolio acute flaccid paralysis.
* Data as of February 21, 2019.
† Per 100,000 children aged <15 years per year.
§ For all subnational areas regardless of population size.
¶ Standard WHO target is adequate stool specimen collection from ≥80% of AFP cases,
assessed by timeliness and condition. For this analysis, timeliness was defined as
two specimens collected ≥24 hours apart (≥1 calendar day in this data set) and within
14 days of paralysis onset. Good condition was defined as arrival of specimens in
a WHO-accredited laboratory with reverse cold chain maintained and without leakage
or desiccation.
** Percentage of the country’s population living in subnational areas that met both
surveillance indicators (NPAFP rates ≥2 per 100,000 children aged <15 years per year
and ≥80% of AFP cases with adequate specimens).
†† cVDPV was associated with at least one case of AFP with evidence of community transmission
and genetically linked. Guidelines for classification of cVDPV can be found at http://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf.
¶¶ Dashes indicate that no confirmed cases were detected.
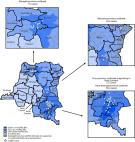
FIGURE
Combined performance indicators for the quality of acute flaccid paralysis surveillance
in subnational areas of 31 countries identified as Global Polio Eradication Initiative
high-priority countries during 2018–2020 — World Health Organization African, Eastern
Mediterranean, South-East Asia, and Western Pacific regions, 2018
Abbreviation: NPAFP = nonpolio acute flaccid paralysis.
The figure is a map showing the combined performance indicators for the quality of
acute flaccid paralysis surveillance during 2018 in subnational areas of 31 countries
identified as Global Polio Eradication Initiative high-priority countries during 2018–2020
in the World Health Organization African, Eastern Mediterranean, Western Pacific,
and South-East Asia regions.
Among the 21 WHO Eastern Mediterranean Region (EMR) countries, the NPAFP rates in
2017 and 2018 were 8.4 and 9.5 per 100,000 children aged <15 years, respectively,
and the respective percentages of AFP cases with adequate stool specimens in 2017
and 2018 were 88% and 90%. In the two countries with endemic WPV transmission, the
number of WPV1 cases increased in Afghanistan (from 14 in 2017 to 21 in 2018) and
Pakistan (from eight in 2017 to 12 in 2018). In 2017, Syria accounted for all 74 reported
cVDPV2 cases in EMR. In 2018, 12 cVDPV cases were reported in Somalia, including five
cVDPV2 cases, six cVDPV type 3 (cVDPV3) cases, and one coinfection of both cVDPV type
2 and type 3. Among the 11 high-priority EMR countries evaluated, nine (82%) countries
in 2017 and 10 (91%) countries in 2018 met both surveillance indicators nationally;
however, as in AFR, national indicators masked subnational underperformance (Table
1) (Figure).
In the WHO Western Pacific Region, 26 cVDPV type 1 (cVDPV1) cases were reported in
Papua New Guinea in 2018. Papua New Guinea did not meet either surveillance indicator
nationally in 2017, and although the NPAFP rate improved in 2018 (mainly related to
implementation of enhanced AFP surveillance as part of the outbreak response), collection
of adequate stool specimen remained low. In the WHO South-East Asia Region, one cVDPV1
case was reported in Indonesia in 2018. Although Indonesia met both surveillance indicators
nationally in 2017 and 2018, subnational weaknesses in surveillance were substantial
(Table 1) (Figure).
Environmental Surveillance
Environmental surveillance (testing of sewage samples) supplements AFP surveillance
by identifying poliovirus transmission in the absence of detected AFP cases (
3
). The number of environmental surveillance sites increased in Afghanistan, Nigeria,
and Pakistan from 143 in 2017 to 185 in 2018. Environmental surveillance detected
no WPV or cVDPV in Nigeria in 2017; however, 46 cVPDV2 isolates were detected in 2018.
Some had been isolated weeks before cases were confirmed. In 2017, four genetic clusters
(isolates with ≥95% genetic relatedness) of WPV1 were detected in sewage samples from
five provinces in Afghanistan, and seven genetic clusters were detected from 19 districts
in Pakistan. In 2018, three WPV1 genetic clusters were detected in sewage samples
from seven provinces in Afghanistan and in five clusters from 27 districts in Pakistan.
In Pakistan, 16% of sewage samples from 19 districts tested positive for WPV1 in 2017,
and 20% from 27 districts tested positive in 2018. Also in 2018, environmental surveillance
detected one cVDPV2 isolate in Kenya as well as 19 cVDPV2 and 11 cVDPV3 isolates in
Somalia. In Papua New Guinea, environmental surveillance detected seven cVDPV1 isolates
from two provinces in 2018. As part of the GPEI’s global environmental surveillance
expansion plan,
†
environmental surveillance is conducted in 44 countries without active WPV transmission,
including 24 in AFR.
Global Polio Laboratory Network
GPLN consists of 146 quality-assured poliovirus laboratories in the six WHO regions.
GPLN laboratories implement standardized protocols to 1) isolate and identify polioviruses;
2) conduct intratypic differentiation (ITD) to identify WPV, Sabin (vaccine) poliovirus,
and VDPV; and 3) conduct genomic sequencing. Poliovirus transmission pathways are
monitored through analysis of the viral capsid protein (VP1) coding region sequences
from isolates. Standard timeliness indicators specify that laboratories should report
≥80% of poliovirus culture results within 14 days of specimen receipt, ≥80% of ITD
results within 7 days of isolate receipt, and ≥80% of sequencing results within 7
days of ITD result. The combined field and laboratory performance indicator is to
report ITD results for ≥80% of isolates within 60 days of paralysis onset in AFP cases.
The accuracy and quality of testing at GPLN laboratories are monitored through an
annual accreditation program of onsite reviews and proficiency testing (
5
). An accreditation checklist was implemented in 2017 for laboratories testing sewage
samples.
GPLN tested 201,546 stool specimens from AFP cases in 2017 and 190,055 in 2018 (Table
2). WPV1 was isolated in specimens from 22 AFP patients in 2017 and 33 patients in
2018. cVDPVs were isolated from 96 patients in 2017 and 104 patients in 2018. GPLN
laboratories in all regions met timeliness indicators for poliovirus isolation and
ITD. All regions met the overall timeliness indicator for onset to ITD results in
both years except the European and Western Pacific Regions in 2018.
TABLE 2
Number of poliovirus isolates from stool specimens of persons with acute flaccid paralysis
(AFP) and timing of results, by World Health Organization (WHO) region — 2017 and
2018*
WHO region/Year
No. of specimens
No. of poliovirus isolates
% Poliovirus isolation results within 7 days of receipt at laboratory
% ITD results within 7 days of receipt of specimen
% ITD results within 60 days of paralysis onset
Wild†
Sabin§
cVDPV¶
African Region
2017
65,245
0
1,663
22
97
80
98
2018
51,292
0
2,547
65
94
98
96
Americas Region
2017
1,755
0
14
0
83
100
100
2018
1,866
0
47
0
86
100
100
Eastern Mediterranean Region
2017
35,602
22
2,521
74
98
99
97
2018
40,419
33
1,749
12
92
99
97
European Region
2017
3,480
0
73
0
83
92
90
2018
3,274
0
71
0
84
92
62
South-East Asia Region
2017
82,292
0
2,251
0
91
96
99
2018
79,566
0
1,970
1
97
100
99
Western Pacific Region
2017
13,370
0
140
0
96
97
90
2018
13,638
0
348
26
97
99
68
Total**
2017
201,546
22
6,662
96
94
91
98
2018
190,055
33
6,732
104
95
99
95
Abbreviations: cVDPV = circulating vaccine-derived poliovirus; ITD = intratypic differentiation;
VP1 = viral capsid protein.
* Data as of March 4, 2019.
† Number of AFP cases with wild poliovirus isolates.
§ Either 1) concordant Sabin-like results in ITD test and vaccine-derived poliovirus
screening or 2) ≤1% VP1 nucleotide sequence difference compared with Sabin vaccine
virus (≤0.6% for type 2).
¶ For poliovirus types 1 and 3, ≥10 VP1 nucleotide differences from the respective
poliovirus; for poliovirus type 2, ≥6 VP1 nucleotide differences from Sabin type 2
poliovirus.
** For the last three indicators, total represents weighted percentage of regional
performance.
In 2018, South Asia genotype (the only WPV1 genotype circulating globally since 2016)
was detected in Afghanistan and Pakistan, with frequent cross-border transmission
between the two countries. Compared with the previous report (
1
), sequence analysis indicates a reduction in the number of orphan WPV1 isolates (those
with less genetic relatedness [≤98.5% in VP1] to other isolates) from AFP patients,
from three in 2017 to zero in 2018, indicating that gaps in AFP surveillance might
be closing; sensitive surveillance identifies AFP cases with isolates that are closely
related. However, the net genetic diversity of WPV1 isolates in Afghanistan and Pakistan
has remained constant for the last 3 years because of the persistent circulation of
many poliovirus lineages in the reservoirs of these countries. In 2018, cVDPVs, most
with extended divergence from the Sabin strain (genetic relatedness = 94%–98.5% identity),
were isolated from stool specimens of AFP patients and from sewage samples, identifying
nine cVDPV emergences during 2018 in seven countries (Democratic Republic of the Congo,
Indonesia, Mozambique, Niger, Nigeria, Papua New Guinea, and Somalia) (
6
,
7
).
Discussion
Although most of the 31 GPEI high-priority countries evaluated met national-level
AFP performance indicators, considerable variation and deficiencies existed at subnational
levels. No substantial improvements were noted in surveillance indicators for these
31 countries from 2017 to 2018. For most of the evaluated AFR countries, the primary
deficiency was the low percentage of AFP cases with adequate specimens, which is most
often the result of delayed case detection after paralysis onset.
In the three countries with endemic WPV transmission, subnational surveillance performance
indicators have been high for several years, even at the district level. In Nigeria,
no WPV1 was detected during August 2014–July 2016; however, during August–September
2016, WPV1 cases were detected in Borno State. Effective AFP surveillance did not
take place in vast insurgent-held areas of Borno during 2013–2016. Since 2016, more
areas have become accessible, and Nigeria has enhanced case detection and reporting
by community-based informants residing in currently inaccessible areas (
8
). AFR will be considered for WPV-free certification in early 2020, and careful examination
of the extent of quality surveillance will be needed to certify the region WPV-free.
Genomic analyses indicated that the cVDPV1s in Indonesia and Papua New Guinea were
circulating several years before detection. Papua New Guinea has experienced chronic
national and subnational deficiencies in AFP case detection and adequate specimen
collection and transport. Subnational surveillance gaps in Indonesia have been identified
previously (
9
). cVDPV outbreaks in regions with endemic polio and those that are polio-free underscore
the need to maintain sensitive poliovirus surveillance everywhere to rapidly detect
and respond to outbreaks.
AFP surveillance has been complemented by environmental surveillance in high-risk
areas, which has allowed detection of cVDPVs before identification of paralyzed patients,
as well as documentation of continued circulation of WPV1 in the reservoir areas of
Afghanistan and Pakistan despite low-level WPV1 case confirmation. In the long term,
continued environmental surveillance will be needed to monitor for poliovirus circulation
in high-risk areas.
The findings in this report are subject to at least two limitations. First, issues
relating to security, hard-to-reach populations, and other factors could affect AFP
surveillance indicators and limit their interpretation. Second, high NPAFP rates do
not necessarily indicate highly sensitive surveillance because not all cases reported
as AFP cases meet the AFP definition and some actual AFP cases might not be detected
by weak surveillance systems.
Strong AFP surveillance, which is essential for global certification of polio eradication,
includes timely case detection, notification, and investigation as well as adequate
stool collection and transport (
10
). External technical and financial support to enhance surveillance has been provided
to all seven countries with cVDPV outbreaks and to the other 24 high-priority countries.
The Global Polio Surveillance Action Plan, 2018–2020 (
4
), specifies which tasks are to be undertaken at the country level; support is tailored
to countries’ needs. Routine monitoring of AFP surveillance performance indicators
at subnational levels and supervision of active surveillance by field personnel are
critical to achieving sensitive poliovirus surveillance. Leading up to certification
of WPV eradication, integrating AFP surveillance with surveillance for other vaccine-preventable
and outbreak-prone diseases will have the advantage of maximizing field surveillance
capacity and performance (
10
).
Summary
What is already known about this topic?
Sensitive acute flaccid paralysis surveillance is the cornerstone of polio eradication
programs.
What is added by this report?
This report presents 2017–2018 poliovirus surveillance data, focusing on 31 countries
identified as high-priority countries by the Global Polio Eradication Initiative.
In 2018, 26 (84%) of the 31 countries met acute flaccid paralysis surveillance indicators
nationally; however, subnational variation in surveillance performance was substantial,
and no improvements were noted from 2017 to 2018.
What are the implications for public health practice?
Surveillance systems need continued strengthening through monitoring, supervision,
and improvements in specimen collection and transport to provide sufficient evidence
for interruption of poliovirus circulation.
Related collections
Most cited references6
- Record: found
- Abstract: found
- Article: not found
Environmental surveillance for polioviruses in the Global Polio Eradication Initiative.
Humayun Asghar, Ousmane Diop, Goitom G Weldegebriel … (2014)
- Record: found
- Abstract: found
- Article: found
Update on Vaccine-Derived Poliovirus Outbreaks — Democratic Republic of the Congo and Horn of Africa, 2017–2018
Chukwuma Mbaeyi, Mary Alleman, Derek Ehrhardt … (2019)

- Record: found
- Abstract: found
- Article: found
Progress Toward Poliomyelitis Eradication — Nigeria, January–December 2017
Omotayo Bolu, Chimeremma Nnadi, Eunice Damisa … (2018)