- Record: found
- Abstract: found
- Article: not found
The endothelial glycocalyx: composition, functions, and visualization
Read this article at
Abstract
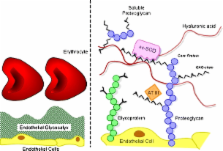
This review aims at presenting state-of-the-art knowledge on the composition and functions of the endothelial glycocalyx. The endothelial glycocalyx is a network of membrane-bound proteoglycans and glycoproteins, covering the endothelium luminally. Both endothelium- and plasma-derived soluble molecules integrate into this mesh. Over the past decade, insight has been gained into the role of the glycocalyx in vascular physiology and pathology, including mechanotransduction, hemostasis, signaling, and blood cell–vessel wall interactions. The contribution of the glycocalyx to diabetes, ischemia/reperfusion, and atherosclerosis is also reviewed. Experimental data from the micro- and macrocirculation alludes at a vasculoprotective role for the glycocalyx. Assessing this possible role of the endothelial glycocalyx requires reliable visualization of this delicate layer, which is a great challenge. An overview is given of the various ways in which the endothelial glycocalyx has been visualized up to now, including first data from two-photon microscopic imaging.
Related collections
Most cited references129
- Record: found
- Abstract: found
- Article: not found
Order out of chaos: assembly of ligand binding sites in heparan sulfate.
- Record: found
- Abstract: found
- Article: not found
Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site.
- Record: found
- Abstract: found
- Article: not found
