- Record: found
- Abstract: found
- Article: not found
Pharmacokinetic‐based failure of a detergent virucidal for severe acute respiratory syndrome–coronavirus‐2 (SARS‐CoV‐2) nasal infections: A preclinical study and randomized controlled trial
Abstract
Background
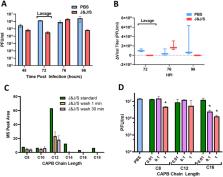
The nose is the portal for severe acute respiratory syndrome–coronavirus‐2 (SARS‐CoV‐2) infection, suggesting the nose as a target for topical antiviral therapies. The purpose of this study was to assess both the in vivo and in vitro efficacy of a detergent‐based virucidal agent, Johnson and Johnson's Baby Shampoo (J&J), in SARS‐CoV‐2–infected subjects.
Methods
Subjects were randomized into three treatment groups: (1) twice daily nasal irrigation with J&J in hypertonic saline, (2) hypertonic saline alone, and (3) no intervention. Complementary in vitro experiments were performed in cultured human nasal epithelia. The primary outcome measure in the clinical trial was change in SARS‐CoV‐2 viral load over 21 days. Secondary outcomes included symptom scores and change in daily temperature. Outcome measures for in vitro studies included change in viral titers.
Results
Seventy‐two subjects completed the clinical study ( n = 24 per group). Despite demonstrated safety and robust efficacy in in vitro virucidal assays, J&J irrigations had no impact on viral titers or symptom scores in treated subjects relative to controls. Similar findings were observed administering J&J to infected cultured human airway epithelia using protocols mimicking the clinical trial regimen. Additional studies of cultured human nasal epithelia demonstrated that lack of efficacy reflected pharmacokinetic failure, with the most virucidal J&J detergent components rapidly absorbed from nasal surfaces.
Conclusion
In this randomized clinical trial of subjects with SARS‐CoV‐2 infection, a topical detergent‐based virucidal agent had no effect on viral load or symptom scores. Complementary in vitro studies confirmed a lack of efficacy, reflective of pharmacokinetic failure and rapid absorption from nasal surfaces.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract

- Record: found
- Abstract: found
- Article: found
Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study
- Record: found
- Abstract: found
- Article: not found
High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.