- Record: found
- Abstract: found
- Article: found
Effect of substrate stiffness on friction in collective cell migration
Read this article at
Abstract
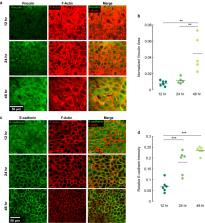
In collective cell migration, the motion results from forces produced by each cell and transmitted to the neighboring cells and to the substrate. Because inertia is negligible and the migration occurs over long time scales, the cell layer exhibits viscous behavior, where force and motion are connected by an apparent friction that results from the breaking and forming of adhesive bonds at the cell–cell and cell–substrate interfaces. Most theoretical models for collective migration include an apparent friction to connect force and motion, with many models making predictions that depend on the ratio of cell–cell and cell–substrate friction. However, little is known about factors that affect friction, leaving predictions of many theoretical models untested. Here, we considered how substrate stiffness and the number of adhesions affected friction at the cell–substrate interface. The experimental data were interpreted through prior theoretical models, which led to the same conclusion, that increased substrate stiffness increased the number of cell–substrate adhesions and caused increased cell–substrate friction. In turn, the friction affected the collective migration by altering the curvature at the edge of the cell layer. By revealing underlying factors affecting friction and demonstrating how friction perturbs the collective migration, this work provides experimental evidence supporting prior theoretical models and motivates the study of other ways to alter the collective migration by changing friction.
Related collections
Most cited references61
- Record: found
- Abstract: not found
- Article: not found
Hydrodynamics of soft active matter
- Record: found
- Abstract: found
- Article: not found
Cell locomotion and focal adhesions are regulated by substrate flexibility.
- Record: found
- Abstract: found
- Article: not found