- Record: found
- Abstract: found
- Article: found
Anti-Inflammatory Effects of Lactobacillus Rahmnosus and Bifidobacterium Breve on Cigarette Smoke Activated Human Macrophages
Read this article at
Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a major global health problem with cigarette smoke (CS) as the main risk factor for its development. Airway inflammation in COPD involves the increased expression of inflammatory mediators such as CXCL-8 and IL-1β which are important mediators for neutrophil recruitment. Macrophages are an important source of these mediators in COPD. Lactobacillus rhamnosus ( L. rhamnosus) and Befidobacterium breve ( B. breve) attenuate the development of ‘allergic asthma’ in animals but their effects in COPD are unknown.
Objective
To determine the anti-inflammatory effects of L. rhamnosus and B. breve on CS and Toll-like receptor (TLR) activation.
Design
We stimulated the human macrophage cell line THP-1 with CS extract in the presence and absence of L. rhamnosus and B. breve and measured the expression and release of inflammatory mediators by RT-qPCR and ELISA respectively. An activity assay and Western blotting were used to examine NF-κB activation.
Results
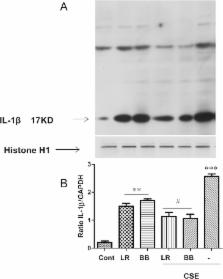
Both L. rhamnosus and B. breve were efficiently phagocytized by human macrophages. L. rhamnosus and B. breve significantly suppressed the ability of CS to induce the expression of IL-1β, IL-6, IL-10, IL-23, TNFα, CXCL-8 and HMGB1 release (all p<0.05) in human THP-1 macrophages. Similar suppression of TLR4- and TLR9-induced CXCL8 expression was also observed (p<0.05). The effect of L. rhamnosus and B. breve on inflammatory mediator release was associated with the suppression of CS-induced NF-κB activation (p<0.05).
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Mechanisms of probiotic actions - A review.
- Record: found
- Abstract: found
- Article: not found
Recognition and signaling by toll-like receptors.
- Record: found
- Abstract: found
- Article: not found