- Record: found
- Abstract: found
- Article: not found
Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics
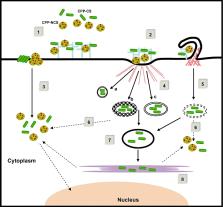
Abstract
The recent discovery of new potent therapeutic molecules that do not reach the clinic due to poor delivery and low bioavailability have made of delivery a key stone in therapeutic development. Several technologies have been designed to improve cellular uptake of therapeutic molecules, including cell-penetrating peptides (CPPs). CPPs were first discovered based on the potency of several proteins to enter cells. Numerous CPPs have been described so far, which can be grouped into two major classes, the first requiring chemical linkage with the drug for cellular internalization and the second involving formation of stable, non-covalent complexes with drugs. Nowadays, CPPs constitute very promising tools for non-invasive cellular import of cargo and have been successfully applied for in vitro and in vivo delivery of therapeutic molecules varying from small chemical molecule, nucleic acids, proteins, peptides, liposomes and particles. This review will focus on the structure/function and cellular uptake mechanism of CPPs in the general context of drug delivery. We will also highlight the application of peptide carriers for the delivery of therapeutic molecules and provide an update of their clinical evaluation.
This article is part of a themed section on Vector Design and Drug Delivery. For a list of all articles in this section see the end of this paper, or visit: http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Related collections
Most cited references132
- Record: found
- Abstract: found
- Article: not found
Regulated portals of entry into the cell.
- Record: found
- Abstract: found
- Article: not found
In vivo protein transduction: delivery of a biologically active protein into the mouse.
- Record: found
- Abstract: found
- Article: not found
