- Record: found
- Abstract: found
- Article: found
Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies
Read this article at
Abstract
Objectives
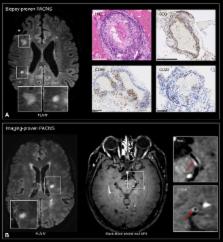
In multiple sclerosis (MS), magnetic resonance imaging (MRI) is a sensitive tool for detecting white matter lesions, but its diagnostic specificity is still suboptimal; ambiguous cases are frequent in clinical practice. Detection of perivenular lesions in the brain (the “central vein sign”) improves the pathological specificity of MS diagnosis, but comprehensive evaluation of this MRI biomarker in MS‐mimicking inflammatory and/or autoimmune diseases, such as central nervous system (CNS) inflammatory vasculopathies, is lacking. In a multicenter study, we assessed the frequency of perivenular lesions in MS versus systemic autoimmune diseases with CNS involvement and primary angiitis of the CNS (PACNS).
Methods
In 31 patients with inflammatory CNS vasculopathies and 52 with relapsing–remitting MS, 3‐dimensional T2*‐weighted and T2–fluid‐attenuated inversion recovery images were obtained during a single MRI acquisition after gadolinium injection. For each lesion, the central vein sign was evaluated according to consensus guidelines. For each patient, lesion count, volume, and brain location, as well as fulfillment of dissemination in space MRI criteria, were assessed.
Results
MS showed higher frequency of perivenular lesions (median = 88%) than did inflammatory CNS vasculopathies (14%), without overlap between groups or differences between 3T and 1.5T MRI. Among inflammatory vasculopathies, Behçet disease showed the highest median frequency of perivenular lesions (34%), followed by PACNS (14%), antiphospholipid syndromes (12%), Sjögren syndrome (11%), and systemic lupus erythematosus (0%). When a threshold of 50% perivenular lesions was applied, central vein sign discriminated MS from inflammatory vasculopathies with a diagnostic accuracy of 100%.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Diagnosis of multiple sclerosis: progress and challenges.
- Record: found
- Abstract: found
- Article: not found
Primary angiitis of the central nervous system.
- Record: found
- Abstract: found
- Article: not found