- Record: found
- Abstract: found
- Article: not found
Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis
Read this article at
Abstract
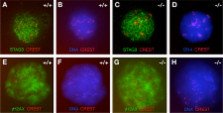
During meiosis, the arrangement of homologous chromosomes is tightly regulated by the synaptonemal complex (SC). Each SC consists of two axial/lateral elements (AEs/LEs), and numerous transverse filaments. SC protein 2 (SYCP2) and SYCP3 are integral components of AEs/LEs in mammals. We find that SYCP2 forms heterodimers with SYCP3 both in vitro and in vivo. An evolutionarily conserved coiled coil domain in SYCP2 is required for binding to SYCP3. We generated a mutant Sycp2 allele in mice that lacks the coiled coil domain. The fertility of homozygous Sycp2 mutant mice is sexually dimorphic; males are sterile because of a block in meiosis, whereas females are subfertile with sharply reduced litter size. Sycp2 mutant spermatocytes exhibit failure in the formation of AEs and chromosomal synapsis. Strikingly, the mutant SYCP2 protein localizes to axial chromosomal cores in both spermatocytes and fetal oocytes, but SYCP3 does not, demonstrating that SYCP2 is a primary determinant of AEs/LEs and, thus, is required for the incorporation of SYCP3 into SCs.
Related collections
Most cited references55
- Record: found
- Abstract: not found
- Article: not found
A drying-down technique for the spreading of mammalian meiocytes from the male and female germline.
- Record: found
- Abstract: found
- Article: not found
The genetics and molecular biology of the synaptonemal complex.
- Record: found
- Abstract: found
- Article: not found
