- Record: found
- Abstract: found
- Article: found
Innate immune modulation in transplantation: mechanisms, challenges, and opportunities
Read this article at
Abstract
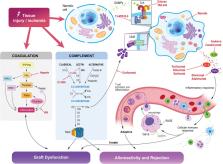
Organ transplantation is characterized by a sequence of steps that involve operative trauma, organ preservation, and ischemia-reperfusion injury in the transplant recipient. During this process, the release of damage-associated molecular patterns (DAMPs) promotes the activation of innate immune cells via engagement of the toll-like receptor (TLR) system, the complement system, and coagulation cascade. Different classes of effector responses are then carried out by specialized populations of macrophages, dendritic cells, and T and B lymphocytes; these play a central role in the orchestration and regulation of the inflammatory response and modulation of the ensuing adaptive immune response to transplant allografts. Organ function and rejection of human allografts have traditionally been studied through the lens of adaptive immunity; however, an increasing body of work has provided a more comprehensive picture of the pivotal role of innate regulation of adaptive immune responses in transplant and the potential therapeutic implications. Herein we review literature that examines the repercussions of inflammatory injury to transplantable organs. We highlight novel concepts in the pathophysiology and mechanisms involved in innate control of adaptive immunity and rejection. Furthermore, we discuss existing evidence on novel therapies aimed at innate immunomodulation and how this could be harnessed in the transplant setting.
Related collections
Most cited references130
- Record: found
- Abstract: found
- Article: not found
Origin and physiological roles of inflammation.
- Record: found
- Abstract: found
- Article: not found
Mechanisms of fibrosis: therapeutic translation for fibrotic disease.
- Record: found
- Abstract: found
- Article: not found