- Record: found
- Abstract: found
- Article: found
Urethral meatus stricture BOO stimulates bladder smooth muscle cell proliferation and pyroptosis via IL-1β and the SGK1-NFAT2 signaling pathway
Read this article at
Abstract
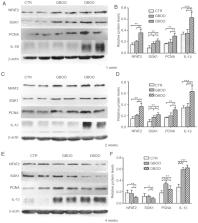
Bladder outlet obstruction (BOO), which is primarily caused by benign prostatic hyperplasia, is a common chronic disease. However, previous studies have most commonly investigated BOO using the acute obstruction model. In the present study, a chronic obstruction model was established to investigate the different pathological alterations in the bladder between acute and chronic obstruction. Compared with chronic obstruction, acute obstruction led to increased expression of proliferating cell nuclear antigen and interleukin-1β, which are markers of proliferation and inflammation, respectively. Furthermore, increased fibrosis in the bladder at week 2 was observed. Low pressure promoted mice bladder smooth muscle cell (MBSMC) proliferation, and pressure overload inhibited cell proliferation and increased the proportion of dead MBSMCs. Further investigation using serum/glucocorticoid regulated kinase 1 (SGK1) small interfering RNAs indicated that low pressure may promote MBSMC proliferation by upregulating SGK1 and nuclear factor of activated T-cell expression levels. Therefore, the present study suggested that acute obstruction led to faster decompensation of bladder function and chronic bladder obstruction displayed an enhanced ability to progress to BOO.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Prognostic value of urodynamic testing in myelodysplastic patients.

- Record: found
- Abstract: found
- Article: found
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis

- Record: found
- Abstract: found
- Article: found