- Record: found
- Abstract: found
- Article: found
Preclinical biodistribution, tropism, and efficacy of oligotropic AAV/Olig001 in a mouse model of congenital white matter disease

Read this article at
Abstract
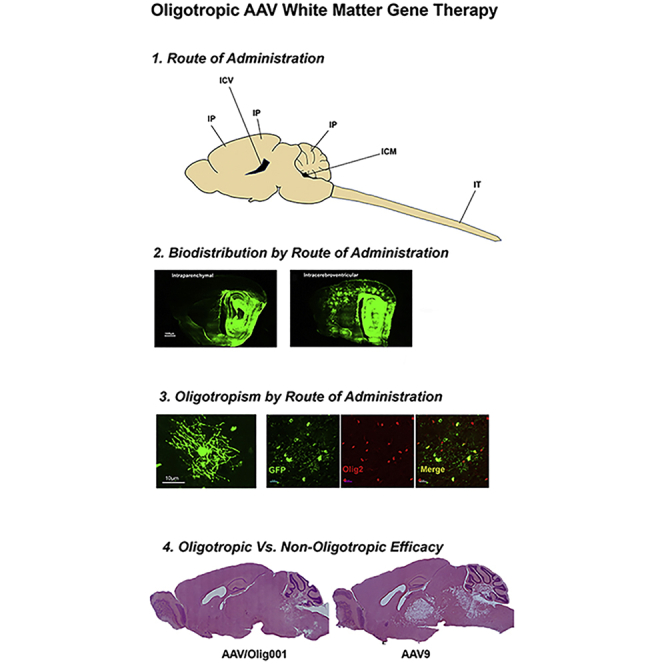
Recent advances in adeno-associated viral (AAV) capsid variants with novel oligotropism require validation in models of disease in order to be viable candidates for white matter disease gene therapy. We present here an assessment of the biodistribution, tropism, and efficacy of a novel AAV capsid variant (AAV/ Olig001) in a model of Canavan disease. We first define a combination of dose and route of administration of an AAV/Olig001-GFP reporter conducive to widespread CNS oligodendrocyte transduction in acutely symptomatic animals that model the Canavan brain at time of diagnosis. Administration of AAV/Olig001-GFP resulted in >70% oligotropism in all regions of interest except the cerebellum without the need for lineage-specific expression elements. Intracerebroventricular infusion into the cerebrospinal fluid (CSF) was identified as the most appropriate route of administration and employed for delivery of an AAV/Olig001 vector to reconstitute oligodendroglial aspartoacylase (ASPA) in adult Canavan mice, which resulted in a dose-dependent rescue of ASPA activity, motor function, and a near-total reduction in vacuolation. A head-to-head efficacy comparison with astrogliotropic AAV9 highlighted a significant advantage conferred by oligotropic AAV/Olig001 that was independent of overall transduction efficiency. These results support the continued development of AAV/Olig001 for advancement to clinical application to white matter disease.
Graphical Abstract
Abstract
This study documents the unique target engagement features of a novel viral vector (AAV/Olig001) that promotes phenotypic rescue in a preclinical model of a white matter disease caused by an inherited defect in a white matter cell enzyme.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator.
- Record: found
- Abstract: found
- Article: not found
NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS.
- Record: found
- Abstract: found
- Article: not found