- Record: found
- Abstract: found
- Article: found
Cerebrospinal fluid tracer efflux to parasagittal dura in humans
Read this article at
Abstract
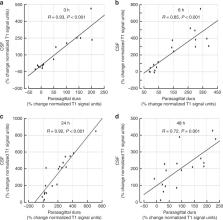
The mechanisms behind molecular transport from cerebrospinal fluid to dural lymphatic vessels remain unknown. This study utilized magnetic resonance imaging along with cerebrospinal fluid tracer to visualize clearance pathways to human dural lymphatics in vivo. In 18 subjects with suspicion of various types of cerebrospinal fluid disorders, 3D T2-Fluid Attenuated Inversion Recovery, T1-black-blood, and T1 gradient echo acquisitions were obtained prior to intrathecal administration of the contrast agent gadobutrol (0.5 ml, 1 mmol/ml), serving as a cerebrospinal fluid tracer. Propagation of tracer was followed with T1 sequences at 3, 6, 24 and 48 h after the injection. The tracer escaped from cerebrospinal fluid into parasagittal dura along the superior sagittal sinus at areas nearby entry of cortical cerebral veins. The findings demonstrate that trans-arachnoid molecular passage does occur and suggest that parasagittal dura may serve as a bridging link between human brain and dural lymphatic vessels.
Abstract
Mechanisms behind molecular transport from cerebrospinal fluid to dural lymphatic vessels remain unknown. This study demonstrates that trans-arachnoid molecular passage does occur and suggests that parasagittal dura may serve as a bridging link between human brain and dural lymphatic vessels.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β.
- Record: found
- Abstract: found
- Article: not found
Structural and functional features of central nervous system lymphatics

- Record: found
- Abstract: found
- Article: found