- Record: found
- Abstract: found
- Article: found
Molecular Mechanisms of Oxytocin Signaling at the Synaptic Connection
Read this article at
Abstract
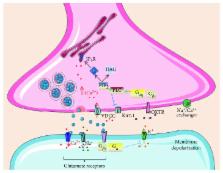
Aberrant regulation of oxytocin signaling is associated with the etiology of neurodevelopmental disorders. Synaptic dysfunctions in neurodevelopmental disorders are becoming increasingly known, and their pathogenic mechanisms could be a target of potential therapeutic intervention. Therefore, it is important to pay attention to the role of oxytocin and its receptor in synapse structure, function, and neuron connectivity. An early alteration in oxytocin signaling may disturb neuronal maturation and may have short-term and long-term pathological consequences. At the molecular level, neurodevelopmental disorders include alterations in cytoskeletal rearrangement and neuritogenesis resulting in a diversity of synaptopathies. The presence of oxytocin receptors in the presynaptic and postsynaptic membranes and the direct effects of oxytocin on neuronal excitability by regulating the activity of ion channels in the cell membrane implicate that alterations in oxytocin signaling could be involved in synaptopathies. The ability of oxytocin to modulate neurogenesis, synaptic plasticity, and certain parameters of cytoskeletal arrangement is discussed in the present review.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Neuropeptide transmission in brain circuits.
- Record: found
- Abstract: found
- Article: not found
Oxytocin Enables Maternal Behavior by Balancing Cortical Inhibition
- Record: found
- Abstract: found
- Article: not found