- Record: found
- Abstract: found
- Article: found
Anxiolytic and Anxiogenic? How the Transcription Factor MEF2 Might Explain the Manifold Behavioral Effects of Oxytocin
Read this article at
Abstract
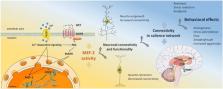
The neuromodulator oxytocin, since its first synthesis by du Vigneaud in 1953, has mainly been associated with beneficial physiological effects, as well as positive social and emotional behaviors. This overall positive picture of oxytocin as the “love-, cuddle-, or bonding-hormone” has repeatedly been challenged since then. Oxytocin-induced effects that would be perceived as negative by the individual, such as increased anxiety or potentiation of stress-induced ACTH release, as well as the regulation of negative approach-related emotions, such as envy and schadenfreude (gloating) have been described. The general consent is that oxytocin, instead of acting unidirectional, induces changes in the salience network to shift the emphasis of emotional contexts, and therefore can, e.g., produce both anxiolytic as well as anxiogenic behavioral outcomes. However, the underlying mechanisms leading to alterations in the salience network are still unclear. With the aim to understand the manifold effects of oxytocin on a cellular/molecular level, a set of oxytocin receptor-coupled signaling cascades and downstream effectors regulating transcription and translation has been identified. Those oxytocin-driven effectors, such as MEF2 and CREB, are known modulators of the neuronal and glial cytoarchitecture. We hypothesize that, by determining cellular morphology and connectivity, MEF2 is one of the key factors that might contribute to the diverse behavioral effects of oxytocin.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication.
- Record: found
- Abstract: found
- Article: not found
Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system.
- Record: found
- Abstract: found
- Article: not found