- Record: found
- Abstract: found
- Article: found
The role of inflammation and genetics in periodontal disease
Read this article at
Abstract
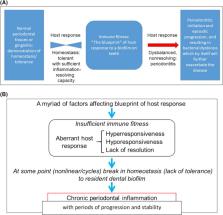
Periodontitis is a complex disease: (a) various causative factors play a role simultaneously and interact with each other; and (b) the disease is episodic in nature, and bursts of disease activity can be recognized, ie, the disease develops and cycles in a nonlinear fashion. We recognize that various causative factors determine the immune blueprint and, consequently, the immune fitness of a subject. Normally, the host lives in a state of homeostasis or symbiosis with the oral microbiome; however, disturbances in homeostatic balance can occur, because of an aberrant host response (inherited and/or acquired during life). This imbalance results from hyper‐ or hyporesponsiveness and/or lack of sufficient resolution of inflammation, which in turn is responsible for much of the disease destruction seen in periodontitis. The control of this destruction by anti‐inflammatory processes and proresolution processes limits the destruction to the tissues surrounding the teeth. The local inflammatory processes can also become systemic, which in turn affect organs such as the heart. Gingival inflammation also elicits changes in the ecology of the subgingival environment providing optimal conditions for the outgrowth of gram‐negative, anaerobic species, which become pathobionts and can propagate periodontal inflammation and can further negatively impact immune fitness. The factors that determine immune fitness are often the same factors that determine the response to the resident biofilm, and are clustered as follows: (a) genetic and epigenetic factors; (b) lifestyle factors, such as smoking, diet, and psychosocial conditions; (c) comorbidities, such as diabetes; and (d) local and dental factors, as well as randomly determined factors (stochasticity). Of critical importance are the pathobionts in a dysbiotic biofilm that drive the viscious cycle. Focusing on genetic factors, currently variants in at least 65 genes have been suggested as being associated with periodontitis based on genome‐wide association studies and candidate gene case control studies. These studies have found pleiotropy between periodontitis and cardiovascular diseases. Most of these studies point to potential pathways in the pathogenesis of periodontal disease. Also, most contribute to a small portion of the total risk profile of periodontitis, often limited to specific racial and ethnic groups. To date, 4 genetic loci are shared between atherosclerotic cardiovascular diseases and periodontitis, ie, CDKN2B‐ AS1( ANRIL ) , a conserved noncoding element within CAMTA1 upstream of VAMP3, PLG, and a haplotype block at the VAMP 8 locus. The shared genes suggest that periodontitis is not causally related to atherosclerotic diseases, but rather both conditions are sequelae of similar (the same?) aberrant inflammatory pathways. In addition to variations in genomic sequences, epigenetic modifications of DNA can affect the genetic blueprint of the host responses. This emerging field will yield new valuable information about susceptibility to periodontitis and subsequent persisting inflammatory reactions in periodontitis. Further studies are required to verify and expand our knowledge base before final cause and effect conclusions about the role of inflammation and genetic factors in periodontitis can be made.
Related collections
Most cited references137
- Record: found
- Abstract: found
- Article: not found
The role of inflammation in depression: from evolutionary imperative to modern treatment target.
- Record: found
- Abstract: found
- Article: not found
Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk.
- Record: found
- Abstract: found
- Article: not found