- Record: found
- Abstract: found
- Article: not found
High-sensitivity next-generation sequencing MRD assessment in ALL identifies patients at very low risk of relapse

Read this article at
Key Points
Visual Abstract
Abstract
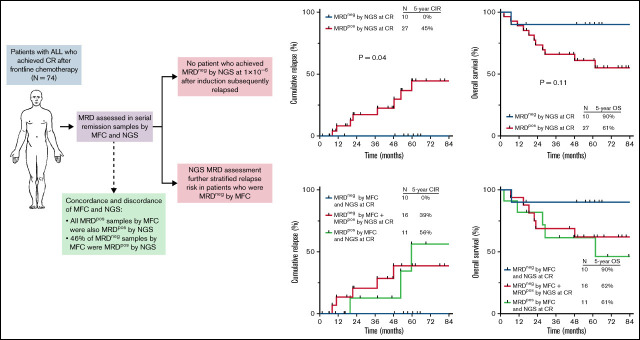
Measurable residual disease (MRD) is highly prognostic for relapse and overall survival (OS) in acute lymphoblastic leukemia (ALL), although many patients with apparent “MRD negativity” by standard assays still relapse. We evaluated the clinical impact of a highly sensitive next-generation sequencing (NGS) MRD assay in 74 adults with ALL undergoing frontline therapy. Among remission samples that were MRD negative by multiparameter flow cytometry (MFC), 46% were MRD + by the NGS assay. After 1 cycle of induction chemotherapy, MRD negativity by MFC at a sensitivity of 1 × 10 −4 and NGS at a sensitivity of 1 × 10 −6 was achieved in 66% and 23% of patients, respectively. The 5-year cumulative incidence of relapse (CIR) among patients who achieved MRD negativity by MFC at complete remission (CR) was 29%; in contrast, no patients who achieved early MRD negativity by NGS relapsed, and their 5-year OS was 90%. NGS MRD negativity at CR was associated with significantly decreased risk of relapse compared with MRD positivity (5-year CIR, 0% vs 45%, respectively; P = .04). Among patients who were MRD negative by MFC, detection of low levels of MRD by NGS identified patients who still had a significant risk of relapse (5-year CIR, 39%). Early assessment of MRD using a highly sensitive NGS assay adds clinically relevant prognostic information to standard MFC-based approaches and can identify patients with ALL undergoing frontline therapy who have a very low risk of relapse and excellent long-term survival.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia.
- Record: found
- Abstract: found
- Article: not found
Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis.
- Record: found
- Abstract: found
- Article: not found