- Record: found
- Abstract: found
- Article: found
Bioactive Natural Spirolactone-Type 6,7- seco- ent-Kaurane Diterpenoids and Synthetic Derivatives
Read this article at
Abstract
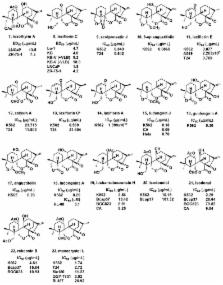
Diterpenoids are widely distributed natural products and have caused considerable interest because of their unique skeletons and antibacterial and antitumor activities and so on. In light of recent discoveries, ent-kaurane diterpenoids, which exhibit a wide variety of biological activities, such as anticancer and anti-inflammatory activities, pose enormous potential to serve as a promising candidate for drug development. Among them, spirolactone-type 6,7- seco- ent-kaurane diterpenoids, with interesting molecular skeleton, complex oxidation patterns, and bond formation, exhibit attractive activities. Furthermore, spirolactone-type diterpenoids have many modifiable sites, which allows for linking to various substituents, suitable for further medicinal study. Hence, some structurally modified derivatives with improved cytotoxicity activities are also achieved. In this review, natural bioactive spirolactone-type diterpenoids and their synthetic derivatives were summarized.
Related collections
Most cited references85
- Record: found
- Abstract: found
- Article: not found
Counting on natural products for drug design.

- Record: found
- Abstract: found
- Article: found
Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer
- Record: found
- Abstract: found
- Article: not found