- Record: found
- Abstract: found
- Article: found
Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa
Kevin D. Hyde ,
Yang Dong ,
Rungtiwa Phookamsak ,
Rajesh Jeewon ,
D. Jayarama Bhat ,
E. B. Gareth Jones ,
Ning-Guo Liu ,
Pranami D. Abeywickrama ,
Ausana Mapook ,
Deping Wei ,
Rekhani H. Perera ,
Ishara S. Manawasinghe ,
Dhandevi Pem ,
Digvijayini Bundhun ,
Anuruddha Karunarathna ,
Anusha H. Ekanayaka ,
Dan-Feng Bao ,
Junfu Li ,
Milan C. Samarakoon ,
Napalai Chaiwan ,
Chuan-Gen Lin ,
Kunthida Phutthacharoen ,
Sheng-Nan Zhang ,
Indunil C. Senanayake ,
Ishani D. Goonasekara ,
Kasun M. Thambugala ,
Chayanard Phukhamsakda ,
Danushka S. Tennakoon ,
Hong-Bo Jiang ,
Jing Yang ,
Ming Zeng ,
Naruemon Huanraluek ,
Jian-Kui (Jack) Liu ,
Subodini N. Wijesinghe ,
Qing Tian ,
Saowaluck Tibpromma ,
Rashika S. Brahmanage ,
Saranyaphat Boonmee ,
Shi-Ke Huang ,
Vinodhini Thiyagaraja ,
Yong-Zhong Lu ,
Ruvishika S. Jayawardena ,
Wei Dong ,
Er-Fu Yang ,
Sanjay K. Singh ,
Shiv Mohan Singh ,
Shiwali Rana ,
Sneha S. Lad ,
Garima Anand ,
Bandarupalli Devadatha ,
M. Niranjan ,
V. Venkateswara Sarma ,
Kare Liimatainen ,
Begoña Aguirre-Hudson ,
Tuula Niskanen ,
Andy Overall ,
Renato Lúcio Mendes Alvarenga ,
Tatiana Baptista Gibertoni ,
Walter P. Pfliegler ,
Enikő Horváth ,
Alexandra Imre ,
Amanda Lucia Alves ,
Ana Carla da Silva Santos ,
Patricia Vieira Tiago ,
Timur S. Bulgakov ,
Dhanushaka N. Wanasinghe ,
Ali H. Bahkali ,
Mingkwan Doilom ,
Abdallah M. Elgorban ,
Sajeewa S. N. Maharachchikumbura ,
Kunhiraman C. Rajeshkumar ,
Danny Haelewaters ,
Peter E. Mortimer ,
Qi Zhao ,
Saisamorn Lumyong ,
Jianchu Xu ,
Jun Sheng
March 16 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references289
- Record: found
- Abstract: not found
- Article: not found
The magnitude of fungal diversity: the 1.5 million species estimate revisited
David L. Hawksworth (2001)

- Record: found
- Abstract: found
- Article: found
The Colletotrichum gloeosporioides species complex
B.S. Weir, P.R. Johnston, U. Damm (2012)
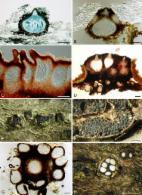
- Record: found
- Abstract: found
- Article: found
The Botryosphaeriaceae: genera and species known from culture
A.J.L. Phillips, A. A. Alves, J. Abdollahzadeh … (2013)