- Record: found
- Abstract: found
- Article: found
SCF ubiquitin E3 ligase regulates DNA double-strand breaks in early meiotic recombination
Read this article at
Abstract
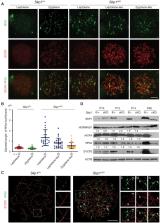
Homeostasis of meiotic DNA double strand breaks (DSB) is critical for germline genome integrity and homologous recombination. Here we demonstrate an essential role for SKP1, a constitutive subunit of the SCF (SKP1-Cullin-F-box) ubiquitin E3 ligase, in early meiotic processes. SKP1 restrains accumulation of HORMAD1 and the pre-DSB complex (IHO1-REC114-MEI4) on the chromosome axis in meiotic germ cells. Loss of SKP1 prior to meiosis leads to aberrant localization of DSB repair proteins and a failure in synapsis initiation in meiosis of both males and females. Furthermore, SKP1 is crucial for sister chromatid cohesion during the pre-meiotic S-phase. Mechanistically, FBXO47, a meiosis-specific F-box protein, interacts with SKP1 and HORMAD1 and targets HORMAD1 for polyubiquitination and degradation in HEK293T cells. Our results support a model wherein the SCF ubiquitin E3 ligase prevents hyperactive DSB formation through proteasome-mediated degradation of HORMAD1 and subsequent modulation of the pre-DSB complex during meiosis.
Related collections
Most cited references78
- Record: found
- Abstract: found
- Article: not found
Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism.
- Record: found
- Abstract: found
- Article: not found
The SCF ubiquitin ligase: insights into a molecular machine.
- Record: found
- Abstract: found
- Article: not found