- Record: found
- Abstract: found
- Article: found
Cellular Senescence in Neurodegenerative Diseases
Read this article at
Abstract
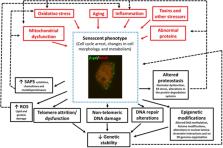
Cellular senescence is a homeostatic biological process characterized by a permanent state of cell cycle arrest that can contribute to the decline of the regenerative potential and function of tissues. The increased presence of senescent cells in different neurodegenerative diseases suggests the contribution of senescence in the pathophysiology of these disorders. Although several factors can induce senescence, DNA damage, oxidative stress, neuroinflammation, and altered proteostasis have been shown to play a role in its onset. Oxidative stress contributes to accelerated aging and cognitive dysfunction stages affecting neurogenesis, neuronal differentiation, connectivity, and survival. During later life stages, it is implicated in the progression of cognitive decline, synapse loss, and neuronal degeneration. Also, neuroinflammation exacerbates oxidative stress, synaptic dysfunction, and neuronal death through the harmful effects of pro-inflammatory cytokines on cell proliferation and maturation. Both oxidative stress and neuroinflammation can induce DNA damage and alterations in DNA repair that, in turn, can exacerbate them. Another important feature associated with senescence is altered proteostasis. Because of the disruption in the function and balance of the proteome, senescence can modify the proper synthesis, folding, quality control, and degradation rate of proteins producing, in some diseases, misfolded proteins or aggregation of abnormal proteins. There is an extensive body of literature that associates cellular senescence with several neurodegenerative disorders including Alzheimer’s disease (AD), Down syndrome (DS), and Parkinson’s disease (PD). This review summarizes the evidence of the shared neuropathological events in these neurodegenerative diseases and the implication of cellular senescence in their onset or aggravation. Understanding the role that cellular senescence plays in them could help to develop new therapeutic strategies.
Related collections
Most cited references280
- Record: found
- Abstract: found
- Article: not found
Cellular senescence in aging and age-related disease: from mechanisms to therapy.
- Record: found
- Abstract: found
- Article: not found
Oxidative stress shortens telomeres.
- Record: found
- Abstract: found
- Article: not found