- Record: found
- Abstract: found
- Article: found
Extracellular Vesicles-Mimetic Encapsulation Improves Oncolytic Viro-Immunotherapy in Tumors With Low Coxsackie and Adenovirus Receptor
Read this article at
Abstract
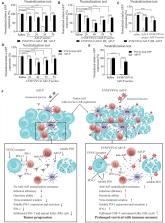
The oncolytic adenovirus (Adv) exhibited poor infection efficiency in tumor cells with low coxsackie and adenovirus receptor (CAR) on the cell surface, which limits the therapeutic efficacy of the Adv-mediated cancer gene therapy. In addition, the abundant adenovirus neutralizing antibodies also abrogate the viral infection of cancer cells. Therefore, novel strategies are required to overcome these two major hurdles to improve the Adv-mediated cancer virotherapy. We constructed a recombinant adenovirus expressing the extracellular domain of PD1 (Ad5-P). The 293T cells expressing VSV-G protein on the cell surface (293T-VSV-G) were infected with Ad5-P. Then Ad5-P infected 293T-VSV-G cells were harvested and squeezed stepwisely through a serial of polycarbonate membranes. Next, the extracellular vesicles-mimetic (EVM) encapsulated Ad5-P (EVM/VSV-G Ad5-P) were collected by density gradient centrifugation. In cell lines with low CAR expression, EVM/VSV-G Ad5-P showed a significantly improved infection efficiency, oncolytic ability, and soluble PD-1 production. In passively immunized mice with Ad5 neutralizing antibody, EVM/VSV-G Ad5-P successfully escaped from antibodies, and the soluble PD-1expression of Ad5-P was significantly prolonged. Finally, EVM/VSV-G Ad5-P treatment significantly improved the antitumor immune responses and prolonged survival of mice with HCC ascites. The EVM/VSV-G Ad5-P not only bypasses the limitation of low CAR expression in tumor cells to improve the viral entry, but also significantly protects the virus from the neutralization antibodies. The EVM encapsulation technology can be successfully used for loading of non-enveloped viruses to generate the extracellular vesicle-mimetic encapsulated viral particles. Our results provide a novel strategy in OVs manufacture to improve the efficacy of tumor oncolytic virotherapy.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Integrating oncolytic viruses in combination cancer immunotherapy
- Record: found
- Abstract: found
- Article: not found
Microvesicle-associated AAV vector as a novel gene delivery system.
- Record: found
- Abstract: found
- Article: not found