- Record: found
- Abstract: found
- Article: found
Visible-light-mediated synthesis of oxime esters via multicomponent reactions of aldehydes, aryl amines, and N-hydroxyphthalimide esters†
Read this article at
Abstract
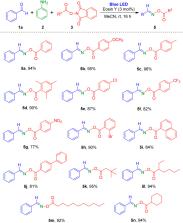
Oxime esters are useful scaffolds in many organic chemistry transformations. Herein, a novel visible-light-mediated three-component reaction for synthesis of oxime esters is reported. Aldehydes, aniline, and N-hydroxyphthalimide (NHPI) esters were used as substrates in this three-component reaction, and eosin Y was used as a crucial photocatalyst for the reaction. Wide ranges of aldehydes and NHPI esters were well tolerated in this reaction method, generating various oxime esters with high efficiency under mild reaction conditions. This visible-light-mediated methodology will be a promising approach to synthesize useful oxime esters in a single step.
Abstract
Practical and efficient visible-light-induced synthesis of oxime esters has been achieved via multicomponent reactions in the presence of catalytic eosin Y.

Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
Organic Photoredox Catalysis.
- Record: found
- Abstract: found
- Article: not found
