- Record: found
- Abstract: found
- Article: found
Principles of Water Electrolysis and Recent Progress in Cobalt‐, Nickel‐, and Iron‐Based Oxides for the Oxygen Evolution Reaction

Read this article at
Abstract
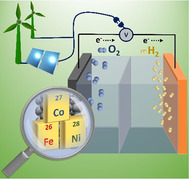
Water electrolysis that results in green hydrogen is the key process towards a circular economy. The supply of sustainable electricity and availability of oxygen evolution reaction (OER) electrocatalysts are the main bottlenecks of the process for large‐scale production of green hydrogen. A broad range of OER electrocatalysts have been explored to decrease the overpotential and boost the kinetics of this sluggish half‐reaction. Co‐, Ni‐, and Fe‐based catalysts have been considered to be potential candidates to replace noble metals due to their tunable 3d electron configuration and spin state, versatility in terms of crystal and electronic structures, as well as abundance in nature. This Review provides some basic principles of water electrolysis, key aspects of OER, and significant criteria for the development of the catalysts. It provides also some insights on recent advances of Co‐, Ni‐, and Fe‐based oxides and a brief perspective on green hydrogen production and the challenges of water electrolysis.
Abstract
This Review describes the basic principles of water electrolysis, key aspects of the oxygen evolution reaction (OER), and significant criteria for the development of new catalysts. Recent advances in catalysts based on Co, Ni, and Fe oxides are described, and a brief perspective is given on green hydrogen production and the challenges of water electrolysis.
Related collections
Most cited references193
- Record: found
- Abstract: found
- Article: not found
Combining theory and experiment in electrocatalysis: Insights into materials design
- Record: found
- Abstract: found
- Article: not found
A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles.
- Record: found
- Abstract: found
- Article: not found