- Record: found
- Abstract: found
- Article: found
Electric-Field-Treated Ni/Co 3O 4 Film as High-Performance Bifunctional Electrocatalysts for Efficient Overall Water Splitting

Read this article at
Abstract
Highlights
-
A novel physical approach is proposed to enhance the electrocatalytic performance by electric field.
-
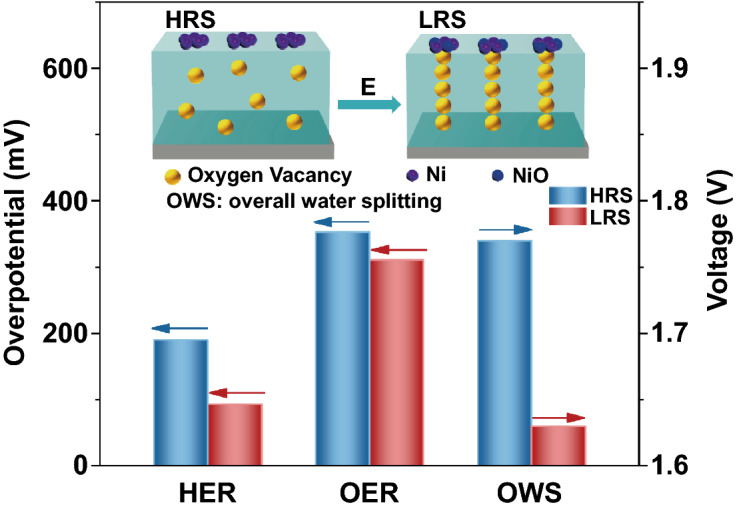
Under the action of electric field, some stable conductive filaments consisting of oxygen vacancies are formed in the Ni/Co 3O 4 film, which remarkably reduces the system resistivity.
-
The electric-field-treated Ni/Co 3O 4 material exhibits significantly superior activity and stability as a bifunctional electrocatalyst for overall water splitting, and its performance exceeds the state-of-the-art electrocatalysts.
Abstract
Rational design of bifunctional electrocatalysts for oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) with excellent activity and stability is of great significance, since overall water splitting is a promising technology for sustainable conversion of clean energy. However, most electrocatalysts do not simultaneously possess optimal HER/OER activities and their electrical conductivities are intrinsically low, which limit the development of overall water splitting. In this paper, a strategy of electric field treatment is proposed and applied to Ni/Co 3O 4 film to develop a novel bifunctional electrocatalyst. After treated by electric field, the conductive channels consisting of oxygen vacancies are formed in the Co 3O 4 film, which remarkably reduces the resistance of the system by almost 2 × 10 4 times. Meanwhile, the surface Ni metal electrode is partially oxidized to nickel oxide, which enhances the catalytic activity. The electric-field-treated Ni/Co 3O 4 material exhibits super outstanding performance of HER, OER, and overall water splitting, and the catalytic activity is significantly superior to the state-of-the-art noble metal catalysts (Pt/C, RuO 2, and RuO 2 ǁ Pt/C couple). This work provides an effective and feasible method for the development of novel and efficient bifunctional electrocatalyst, which is also promising for wide use in the field of catalysis.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
Opportunities and challenges for a sustainable energy future.
- Record: found
- Abstract: found
- Article: not found
Noble metal-free hydrogen evolution catalysts for water splitting.
- Record: found
- Abstract: found
- Article: not found