- Record: found
- Abstract: found
- Article: found
Monocyte-derived macrophage assisted breast cancer cell invasion as a personalized, predictive metric to score metastatic risk
Read this article at
Abstract
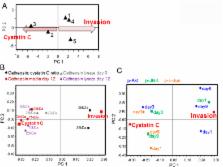
Patient-to-patient variability in breast cancer progression complicates clinical treatment decisions. Of women undergoing prophylactic mastectomies, many may not have progressed to indolent forms of disease and could have benefited from milder, localized therapy. Tumor associated macrophages contribute significantly to tumor invasion and metastasis, with cysteine cathepsin proteases as important contributors. Here, a method is demonstrated by which variability in macrophage expression of cysteine cathepsins, their inhibitor cystatin C, and kinase activation can be used to train a multivariate model and score patients for invasion risk. These enzymatic profiles were used to predict macrophage-assisted MCF-7 breast cancer cell invasion in the trained computational model. To test these predictions, a priori, signals from monocytes isolated from women undergoing mastectomies were input to score their cancer invasion potential in a patient-specific manner, and successfully predicted that patient monocytes with highest predicted invasion indices matched those with more invasive initial diagnoses of the nine patients tested. Together this establishes proof-of-principle that personalized information acquired from minimally invasive blood draws may provide useful information to inform oncologists and patients of invasive/metastatic risk, helping to make decisions regarding radical mastectomy or milder, conservative treatments to save patients from hardship and surgical recovery.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Distinct role of macrophages in different tumor microenvironments.
- Record: found
- Abstract: found
- Article: not found
Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma.
- Record: found
- Abstract: found
- Article: not found