- Record: found
- Abstract: found
- Article: found
MicroRNAs of Epstein-Barr Virus Attenuate T-Cell-Mediated Immune Control In Vivo
Read this article at
Abstract
Epstein-Barr virus (EBV) infects the majority of the human population and usually persists asymptomatically within its host. Nevertheless, EBV is the causative agent for infectious mononucleosis (IM) and for lymphoproliferative disorders, including Burkitt and Hodgkin lymphomas. The immune system of the infected host is thought to prevent tumor formation in healthy virus carriers. EBV was one of the first viruses described to express miRNAs, and many host and viral targets were identified for these in vitro. However, their role during EBV infection in vivo remained unclear. This work is the first to describe that EBV miRNAs mainly increase viremia and virus-associated lymphomas through dampening antigen recognition by adaptive immune responses in mice with reconstituted immune responses. Currently, there is no prophylactic or therapeutic treatment to restrict IM or EBV-associated malignancies; thus, targeting EBV miRNAs could promote immune responses and limit EBV-associated pathologies.
ABSTRACT
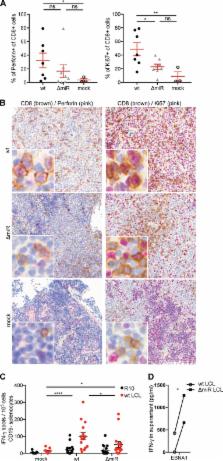
The human persistent and oncogenic Epstein-Barr virus (EBV) was one of the first viruses that were described to express viral microRNAs (miRNAs). These have been proposed to modulate many host and viral functions, but their predominant role in vivo has remained unclear. We compared recombinant EBVs expressing or lacking miRNAs during in vivo infection of mice with reconstituted human immune system components and found that miRNA-deficient EBV replicates to lower viral titers with decreased frequencies of proliferating EBV-infected B cells. In response, activated cytotoxic EBV-specific T cells expand to lower frequencies than during infection with miRNA-expressing EBV. However, when we depleted CD8 + T cells the miRNA-deficient virus reached similar viral loads as wild-type EBV, increasing by more than 200-fold in the spleens of infected animals. Furthermore, CD8 + T cell depletion resulted in lymphoma formation in the majority of animals after miRNA-deficient EBV infection, while no tumors emerged when CD8 + T cells were present. Thus, miRNAs mainly serve the purpose of immune evasion from T cells in vivo and could become a therapeutic target to render EBV-associated malignancies more immunogenic.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Epstein-Barr virus: more than 50 years old and still providing surprises.
- Record: found
- Abstract: found
- Article: not found
Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells.
- Record: found
- Abstract: found
- Article: not found