- Record: found
- Abstract: found
- Article: found
Redirecting T Cells against Epstein–Barr Virus Infection and Associated Oncogenesis
Read this article at
Abstract
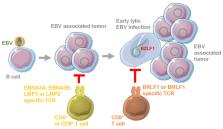
The Epstein–Barr virus (EBV) is associated with lymphomas and carcinomas. For some of these, the adoptive transfer of EBV specific T cells has been therapeutically explored, with clinical success. In order to avoid naturally occurring EBV specific autologous T cell selection from every patient, the transgenic expression of latent and early lytic viral antigen specific T cell receptors (TCRs) to redirect T cells, to target the respective tumors, is being developed. Recent evidence suggests that not only TCRs against transforming latent EBV antigens, but also against early lytic viral gene products, might be protective for the control of EBV infection and associated oncogenesis. At the same time, these approaches might be more selective and cause less collateral damage than targeting general B cell markers with chimeric antigen receptors (CARs). Thus, EBV specific TCR transgenic T cells constitute a promising therapeutic strategy against EBV associated malignancies.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Epstein-Barr virus: exploiting the immune system.
- Record: found
- Abstract: found
- Article: not found
EBV persistence in memory B cells in vivo.
- Record: found
- Abstract: found
- Article: not found