- Record: found
- Abstract: found
- Article: found
Clinical Performance of the Point-of-Care cobas Liat for Detection of SARS-CoV-2 in 20 Minutes: a Multicenter Study
Read this article at
Abstract
Highly accurate testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the point of care (POC) is an unmet diagnostic need in emergency care and time-sensitive outpatient care settings. Reverse transcription-PCR (RT-PCR) technology is the gold standard for SARS-CoV-2 diagnostics.
ABSTRACT
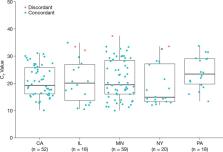
Highly accurate testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the point of care (POC) is an unmet diagnostic need in emergency care and time-sensitive outpatient care settings. Reverse transcription-PCR (RT-PCR) technology is the gold standard for SARS-CoV-2 diagnostics. We performed a multisite U.S. study comparing the clinical performance of the first U.S. Food and Drug Administration (FDA)-authorized POC RT-PCR for detection of SARS-CoV-2 in 20 min, the cobas Liat SARS-CoV-2 and influenza A/B nucleic acid test, to the most widely used RT-PCR laboratory test, the cobas 68/8800 SARS-CoV-2 test. Clinical nasopharyngeal swab specimens from 444 patients with 357 evaluable specimens at five U.S. clinical laboratories were enrolled from 21 September 2020 to 23 October 2020. The overall agreement between the Liat and 68/8800 systems for SARS-CoV-2 diagnostics was 98.6% (352/357). Using Liat, positive percent agreement for SARS-CoV-2 was 100% (162/162) and the negative percent agreement was 97.4% (190/195). The Liat is an RT-PCR POC test that provides highly accurate SARS-CoV-2 results in 20 min with performance equivalent to that of high-throughput laboratory molecular testing. Rapid RT-PCR testing at the POC can enable more timely infection control and individual care decisions for coronavirus disease 2019.
Related collections
Most cited references23

- Record: found
- Abstract: found
- Article: found
Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020
- Record: found
- Abstract: found
- Article: not found
Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19

- Record: found
- Abstract: found
- Article: found