- Record: found
- Abstract: found
- Article: found
A narrative review of methods for the identification of ALK fusions in patients with non-small cell lung carcinoma
Read this article at
Abstract
Background and Objective
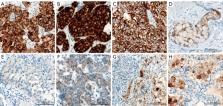
This narrative review is intended to provide pragmatic knowledge of current methods for the search of anaplastic lymphoma kinase ( ALK) fusions in patients with non-small cell lung carcinoma (NSCLC). This information is very timely, because a recent survey has identified that almost 50% of patients with advanced NSCLC were not candidates for targeted therapies because of biomarker testing issues.
Methods
PubMed was searched from January 1 st, 2012 to February 28 th, 2023 using the following keywords: “ ALK” and “lung”, including reviews and our own work.
Key Content and Findings
Testing rates have not reached 85% among patients’ candidates to ALK inhibition. The advantages and disadvantages of the different analytical options [immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), real-time polymerase chain reaction and next-generation sequencing (NGS)] are discussed. The key factor for success in ALK testing is a deep understanding of the concept of “molecular redundancy”. This notion has been recommended and endorsed by all the major professional organizations in the field and can be summarized as follows: “laboratories should ensure that test results that are unexpected, discordant, equivocal, or otherwise of low confidence are confirmed or resolved using an alternative method or sample”. In-depth knowledge of the different ALK testing methodologies can help clinical and molecular tumor boards implement and maintain sensible algorithms for a rapid and effective detection of predictive biomarkers in patients with NSCLC.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
Lung cancer
- Record: found
- Abstract: found
- Article: not found
Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer.
- Record: found
- Abstract: found
- Article: not found