- Record: found
- Abstract: found
- Article: found
Ketamine Alters Functional Plasticity of Astroglia: An Implication for Antidepressant Effect
Read this article at
Abstract
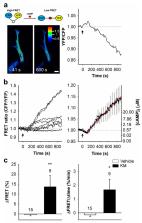
Ketamine, a non-competitive N–methyl– d–aspartate receptor (NMDAR) antagonist, exerts a rapid, potent and long-lasting antidepressant effect, although the cellular and molecular mechanisms of this action are yet to be clarified. In addition to targeting neuronal NMDARs fundamental for synaptic transmission, ketamine also affects the function of astrocytes, the key homeostatic cells of the central nervous system that contribute to pathophysiology of major depressive disorder. Here, I review studies revealing that (sub)anesthetic doses of ketamine elevate intracellular cAMP concentration ([cAMP] i) in astrocytes, attenuate stimulus-evoked astrocyte calcium signaling, which regulates exocytotic secretion of gliosignaling molecules, and stabilize the vesicle fusion pore in a narrow configuration, possibly hindering cargo discharge or vesicle recycling. Next, I discuss how ketamine affects astrocyte capacity to control extracellular K + by reducing vesicular delivery of the inward rectifying potassium channel (K ir4.1) to the plasmalemma that reduces the surface density of Kir4.1. Modified astroglial K + buffering impacts upon neuronal firing pattern as demonstrated in lateral habenula in a rat model of depression. Finally, I highlight the discovery that ketamine rapidly redistributes cholesterol in the astrocyte plasmalemma, which may alter the flux of cholesterol to neurons. This structural modification may further modulate a host of processes that synergistically contribute to ketamine’s rapid antidepressant action.
Related collections
Most cited references197
- Record: found
- Abstract: found
- Article: not found
Astrocyte-endothelial interactions at the blood-brain barrier.
- Record: found
- Abstract: found
- Article: found
Reactive astrocyte nomenclature, definitions, and future directions
- Record: found
- Abstract: found
- Article: not found