- Record: found
- Abstract: found
- Article: not found
Rapid-acting antidepressants and the circadian clock
Read this article at
Abstract
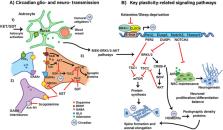
A growing number of epidemiological and experimental studies has established that circadian disruption is strongly associated with psychiatric disorders, including major depressive disorder (MDD). This association is becoming increasingly relevant considering that modern lifestyles, social zeitgebers (time cues) and genetic variants contribute to disrupting circadian rhythms that may lead to psychiatric disorders. Circadian abnormalities associated with MDD include dysregulated rhythms of sleep, temperature, hormonal secretions, and mood which are modulated by the molecular clock. Rapid-acting antidepressants such as subanesthetic ketamine and sleep deprivation therapy can improve symptoms within 24 h in a subset of depressed patients, in striking contrast to conventional treatments, which generally require weeks for a full clinical response. Importantly, animal data show that sleep deprivation and ketamine have overlapping effects on clock gene expression. Furthermore, emerging data implicate the circadian system as a critical component involved in rapid antidepressant responses via several intracellular signaling pathways such as GSK3β, mTOR, MAPK, and NOTCH to initiate synaptic plasticity. Future research on the relationship between depression and the circadian clock may contribute to the development of novel therapeutic strategies for depression-like symptoms. In this review we summarize recent evidence describing: (1) how the circadian clock is implicated in depression, (2) how clock genes may contribute to fast-acting antidepressants, and (3) the mechanistic links between the clock genes driving circadian rhythms and neuroplasticity.
Related collections
Most cited references232
- Record: found
- Abstract: found
- Article: found
Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression
- Record: found
- Abstract: found
- Article: not found
Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration.
- Record: found
- Abstract: found
- Article: not found
