- Record: found
- Abstract: found
- Article: found
Combination therapy (toripalimab and lenvatinib)-associated toxic epidermal necrolysis in a patient with metastatic liver cancer: A case report
Read this article at
Abstract
BACKGROUND
Both programmed cell death-1 (PD-1) inhibitors and lenvatinib, which have a synergistic effect, are promising drugs for tumor treatment. It is generally believed that combination therapy with a PD-1 inhibitor and lenvatinib is safe and effective. However, we report a case of toxic epidermal necrolysis (TEN), a grade 4 toxicity, after this combination therapy.
CASE SUMMARY
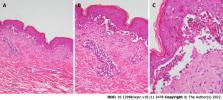
A 39-year-old male presented with erythema, blisters and erosions on the face, neck, trunk and limbs 1 wk after receiving combination therapy with lenvatinib and toripalimab, a PD-1 inhibitor. The skin injury covered more than 70% of the body surface area. He was previously diagnosed with liver cancer with cervical vertebra metastasis. Histologically, prominent necrotic keratinocytes, hyperkeratosis, liquefaction of basal cells and acantholytic bullae were observed in the epidermis. Blood vessels in the dermis were infiltrated by lymphocytes and eosinophils. Direct immunofluorescence staining was negative. Thus, the diagnosis was confirmed to be TEN (associated with combination therapy with toripalimab and lenvatinib). Full-dose and long-term corticosteroids, high-dose intravenous immunoglobulin and targeted antibiotic drugs were administered. The rashes gradually faded; however, as expected, the tumor progressed. Therefore, sorafenib and regorafenib were given in succession, and the patient was still alive at the 10-mo follow-up.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial
- Record: found
- Abstract: not found
- Article: not found
AASLD guidelines for the treatment of hepatocellular carcinoma.

- Record: found
- Abstract: found
- Article: found