- Record: found
- Abstract: found
- Article: found
Ex vivo, in situ perfusion protocol for human brain fixation compatible with microscopy, MRI techniques, and anatomical studies
Read this article at
Abstract
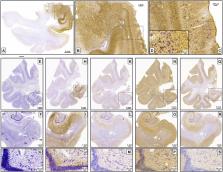
We present a method for human brain fixation based on simultaneous perfusion of 4% paraformaldehyde through carotids after a flush with saline. The left carotid cannula is used to perfuse the body with 10% formalin, to allow further use of the body for anatomical research or teaching. The aim of our method is to develop a vascular fixation protocol for the human brain, by adapting protocols that are commonly used in experimental animal studies. We show that a variety of histological procedures can be carried out (cyto- and myeloarchitectonics, histochemistry, immunohistochemistry, intracellular cell injection, and electron microscopy). In addition, ex vivo, ex situ high-resolution MRI (9.4T) can be obtained in the same specimens. This procedure resulted in similar morphological features to those obtained by intravascular perfusion in experimental animals, provided that the postmortem interval was under 10 h for several of the techniques used and under 4 h in the case of intracellular injections and electron microscopy. The use of intravascular fixation of the brain inside the skull provides a fixed whole human brain, perfectly fitted to the skull, with negligible deformation compared to conventional techniques. Given this characteristic of ex vivo, in situ fixation, this procedure can probably be considered the most suitable one available for ex vivo MRI scans of the brain. We describe the compatibility of the method proposed for intravascular fixation of the human brain and fixation of the donor’s body for anatomical purposes. Thus, body donor programs can provide human brain tissue, while the remainder of the body can also be fixed for anatomical studies. Therefore, this method of human brain fixation through the carotid system optimizes the procurement of human brain tissue, allowing a greater understanding of human neurological diseases, while benefiting anatomy departments by making the remainder of the body available for teaching purposes.
Related collections
Most cited references82

- Record: found
- Abstract: found
- Article: found
A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology
- Record: found
- Abstract: found
- Article: not found
Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol.
- Record: found
- Abstract: found
- Article: not found