- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth
Read this article at
Abstract
Objective
To characterize the demographic and clinical features of pediatric SARS-CoV-2 syndromes and identify admission variables predictive of disease severity.
Study design
We conducted a multicenter, retrospective and prospective study of pediatric patients hospitalized with acute SARS-CoV-2 infections and multisystem inflammatory syndrome in children (MIS-C) at eight sites in New York, New Jersey, and Connecticut.
Results
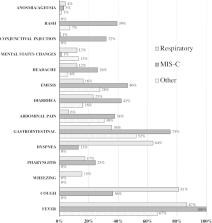
We identified 281 hospitalized patients with SARS-CoV-2 infections and divided them into three groups based on clinical features. Overall, 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations including gastrointestinal illness or fever. Patients with MIS-C were more likely to identify as non-Hispanic black compared with patients with respiratory disease (35% versus 18%, P=.02). Seven patients (2%) died and 114 (41%) were admitted to the ICU. In multivariable analyses, obesity (OR=3.39, 95% CI:1.26-9.10, P=.02) and hypoxia on admission (OR=4.01; 95% CI:1.14-14.15; P=.03) were predictive of severe respiratory disease. Lower absolute lymphocyte count (OR=8.33 per unit decrease in 10 9 cells/L, 95% CI:2.32-33.33, P=.001) and higher C-reactive protein (OR=1.06 per unit increase in mg/dL, 95% CI:1.01-1.12, P=.017) were predictive of severe MIS-C. Race/ethnicity or socioeconomic status were not predictive of disease severity.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
- Record: found
- Abstract: found
- Article: found
Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area
- Record: found
- Abstract: found
- Article: found
