- Record: found
- Abstract: found
- Article: found
Membrane Traffic and Fusion at Post-Golgi Compartments
Read this article at
Abstract
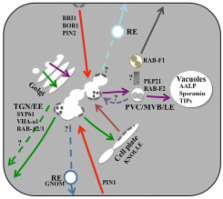
Complete sequencing of the Arabidopsis genome a decade ago has facilitated the functional analysis of various biological processes including membrane traffic by which many proteins are delivered to their sites of action and turnover. In particular, membrane traffic between post-Golgi compartments plays an important role in cell signaling, taking care of receptor–ligand interaction and inactivation, which requires secretion, endocytosis, and recycling or targeting to the vacuole for degradation. Here, we discuss recent studies that address the identity of post-Golgi compartments, the machinery involved in traffic and fusion or functionally characterized cargo proteins that are delivered to or pass through post-Golgi compartments. We also provide an outlook on future challenges in this area of research.
Related collections
Most cited references198
- Record: found
- Abstract: found
- Article: not found
Membrane fusion: grappling with SNARE and SM proteins.
- Record: found
- Abstract: found
- Article: not found
Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis.
- Record: found
- Abstract: found
- Article: not found