- Record: found
- Abstract: found
- Article: found
Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells
Read this article at
Abstract
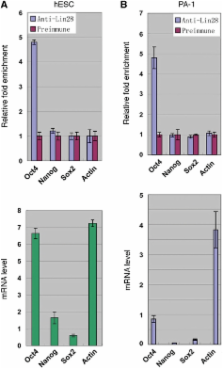
Lin28 acts as a repressor of microRNA processing and as a post-transcriptional regulatory factor for a subset of mRNAs. Here we report that in human embryonic stem cells Lin28 facilitates the expression of the pivotal pluripotency factor Oct4 at the post-transcriptional level. We provide evidence that Lin28 binds Oct4 mRNA directly through high affinity sites within its coding region and that an interaction between Lin28 and RNA helicase A (RHA) may play a part in the observed regulation. We further demonstrate that decreasing RHA levels impairs Lin28-dependent stimulation of translation in a reporter system. Taken together with previous studies showing that RHA is required for efficient translation of a specific class of mRNAs, these findings suggest a novel mechanism by which Lin28 may affect target mRNA expression and represent the first evidence of post-transcriptional regulation of Oct4 expression by Lin28 in human embryonic stem cells.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Selective blockade of microRNA processing by Lin28.
- Record: found
- Abstract: found
- Article: not found
Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA.
- Record: found
- Abstract: found
- Article: not found