- Record: found
- Abstract: found
- Article: found
Monalizumab: inhibiting the novel immune checkpoint NKG2A
Read this article at
Abstract
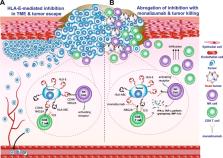
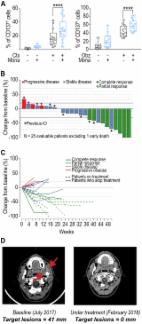
The implementation of immune checkpoint inhibitors to the oncology clinic signified a new era in cancer treatment. After the first indication of melanoma, an increasing list of additional cancer types are now treated with immune system targeting antibodies to PD-1, PD-L1 and CTLA-4, alleviating inhibition signals on T cells. Recently, we published proof-of-concept results on a novel checkpoint inhibitor, NKG2A. This receptor is expressed on cytotoxic lymphocytes, including NK cells and subsets of activated CD8 + T cells. Blocking antibodies to NKG2A unleashed the reactivity of these effector cells resulting in tumor control in multiple mouse models and an early clinical trial. Monalizumab is inhibiting this checkpoint in human beings and future clinical trials will have to reveal its potency in combination with other cancer treatment options.
Related collections
Most cited references49

- Record: found
- Abstract: found
- Article: found
NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control
- Record: found
- Abstract: found
- Article: not found
HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C.
- Record: found
- Abstract: found
- Article: found