- Record: found
- Abstract: found
- Article: found
Hyperparathyroidism Is an Independent Risk Factor for Allograft Dysfunction in Pediatric Kidney Transplantation

Abstract
Introduction
Little is known about the consequences of deranged chronic kidney disease–mineral and bone disorder (CKD-MBD) parameters on kidney allograft function in children. We examined a relationship between these parameters over time and allograft outcome.
Methods
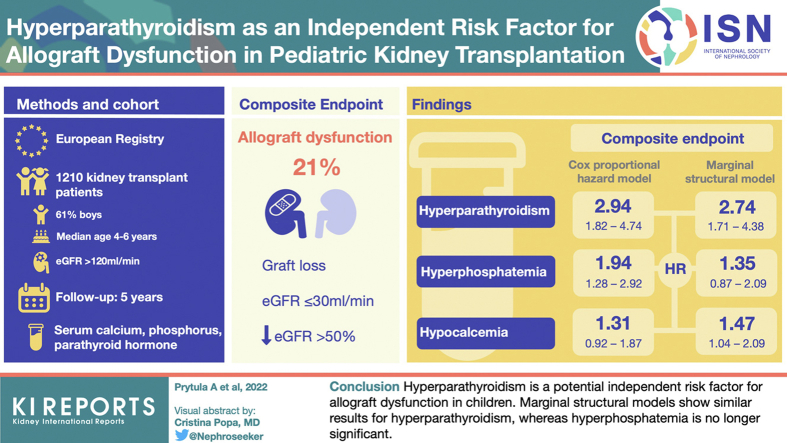
This registry study from the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) collected data at baseline, months 1, 3, 6, 9, and 12 after transplant; and every 6 months thereafter up to 5 years. Survival analysis for a composite end point of graft loss or estimated glomerular filtration rate (eGFR) ≤30 ml/min per 1.73 m 2 or a ≥50% decline from eGFR at month 1 posttransplant was performed. Associations of parathyroid hormone (PTH), calcium, phosphate, and 25-hydroxyvitamin D (25(OH)D) with allograft outcome were investigated using conventional stratified Cox proportional hazards models and further verified with marginal structural models with time-varying covariates.
Results
We report on 1210 patients (61% boys) from 16 European countries. The composite end point was reached in 250 grafts (21%), of which 11 (4%) were allograft losses. In the conventional Cox proportional hazards models adjusted for potential confounders, only hyperparathyroidism (hazard ratio [HR], 2.94; 95% confidence interval [CI], 1.82–4.74) and hyperphosphatemia (HR, 1.94; 95% CI, 1.28–2.92) were associated with the composite end point. Marginal structural models showed similar results for hyperparathyroidism (HR, 2.74; 95% CI, 1.71–4.38), whereas hyperphosphatemia was no longer significant (HR, 1.35; 95% CI, 0.87–2.09), suggesting that its association with graft dysfunction can be ascribed to a decline in eGFR.
Graphical abstract
Related collections
Most cited references38
- Record: found
- Abstract: not found
- Article: not found
Marginal Structural Models and Causal Inference in Epidemiology
- Record: found
- Abstract: found
- Article: not found