- Record: found
- Abstract: found
- Article: found
Development of an Equine Groove Model to Induce Metacarpophalangeal Osteoarthritis: A Pilot Study on 6 Horses
Read this article at
Abstract
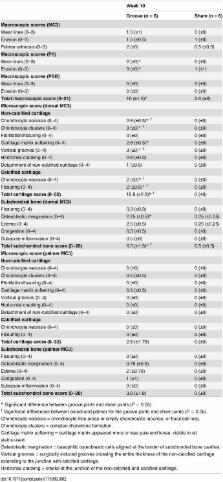
The aim of this work was to develop an equine metacarpophalangeal joint model that induces osteoarthritis that is not primarily mediated by instability or inflammation. The study involved six Standardbred horses. Standardized cartilage surface damage or “grooves” were created arthroscopically on the distal dorsal aspect of the lateral and medial metacarpal condyles of a randomly chosen limb. The contralateral limb was sham operated. After 2 weeks of stall rest, horses were trotted 30 minutes every other day for 8 weeks, then evaluated for lameness and radiographed. Synovial fluid was analyzed for cytology and biomarkers. At 10 weeks post-surgery, horses were euthanized for macroscopic and histologic joint evaluation. Arthroscopic grooving allowed precise and identical damage to the cartilage of all animals. Under the controlled exercise regime, this osteoarthritis groove model displayed significant radiographic, macroscopic, and microscopic degenerative and reactive changes. Histology demonstrated consistent surgically induced grooves limited to non-calcified cartilage and accompanied by secondary adjacent cartilage lesions, chondrocyte necrosis, chondrocyte clusters, cartilage matrix softening, fissuring, mild subchondral bone inflammation, edema, and osteoblastic margination. Synovial fluid biochemistry and cytology demonstrated significantly elevated total protein without an increase in prostaglandin E2, neutrophils, or chondrocytes. This equine metacarpophalangeal groove model demonstrated that standardized non-calcified cartilage damage accompanied by exercise triggered altered osteochondral morphology and cartilage degeneration with minimal or inefficient repair and little inflammatory response. This model, if validated, would allow for assessment of disease processes and the effects of therapy.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene.
- Record: found
- Abstract: found
- Article: not found
Early events in cartilage repair after subchondral bone microfracture.
- Record: found
- Abstract: found
- Article: not found