- Record: found
- Abstract: found
- Article: found
Oxidation state-specific fluorescent copper sensors reveal oncogene-driven redox changes that regulate labile copper(II) pools
Read this article at
Significance
Copper sustains fundamental chemical processes across all kingdoms of life by cycling between two major oxidation states, Cu(I) and Cu(II). However, in contrast to advances in fluorescent sensors and related probes to help decipher Cu(I) biology, Cu(II) detection remains lacking. We present a strategy using metal-directed acyl imidazole chemistry to enable both metal and oxidation state-specific Cu(II) detection by a tandem activity-based sensing/labeling reaction. We use this platform to identify the existence of loosely bound, labile Cu(II) pools, a Cu(II) import protein in cells, and reciprocal Cu(II) elevations and Cu(I) deficiencies driven by a loss of antioxidant defense in cellular models of cancer.
Abstract
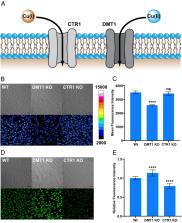
Copper is an essential metal nutrient for life that often relies on redox cycling between Cu(I) and Cu(II) oxidation states to fulfill its physiological roles, but alterations in cellular redox status can lead to imbalances in copper homeostasis that contribute to cancer and other metalloplasias with metal-dependent disease vulnerabilities. Copper-responsive fluorescent probes offer powerful tools to study labile copper pools, but most of these reagents target Cu(I), with limited methods for monitoring Cu(II) owing to its potent fluorescence quenching properties. Here, we report an activity-based sensing strategy for turn-on, oxidation state-specific detection of Cu(II) through metal-directed acyl imidazole chemistry. Cu(II) binding to a metal and oxidation state-specific receptor that accommodates the harder Lewis acidity of Cu(II) relative to Cu(I) activates the pendant dye for reaction with proximal biological nucleophiles and concomitant metal ion release, thus avoiding fluorescence quenching. Copper-directed acyl imidazole 649 for Cu(II) (CD649.2) provides foundational information on the existence and regulation of labile Cu(II) pools, including identifying divalent metal transporter 1 (DMT1) as a Cu(II) importer, labile Cu(II) increases in response to oxidative stress induced by depleting total glutathione levels, and reciprocal increases in labile Cu(II) accompanied by decreases in labile Cu(I) induced by oncogenic mutations that promote oxidative stress.
Related collections
Most cited references81
- Record: found
- Abstract: not found
- Article: not found
Absolute hardness: companion parameter to absolute electronegativity
- Record: found
- Abstract: not found
- Article: not found