- Record: found
- Abstract: found
- Article: not found
Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): Do homo sapiens acquire or have a reward deficiency syndrome?
Read this article at
Abstract
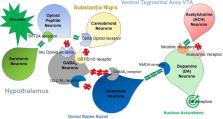
The extant literature confirms that an array of polymorphic genes related to- neurotransmitters and second messengers govern the net release of dopamine in the Nucleus Accumbens (NAc) in the mesolimbic region of the brain. They are linked predominantly to motivation, anti-stress, incentive salience (wanting), and wellbeing. Notably, in 2000 the Nobel Prize was awarded to Carlsson, Greengard, and Kandel for their work on the molecular and cellular function of dopaminergic activity at neurons. This historical psychopharmacological work involved neurotransmission of serotonin, endorphins, glutamate, and dopamine, and the seminal work of Blum, Gold, Volkow, Nestler, and others related to neurotransmitter function and related behaviors. Currently, Americans are facing their second and worst opioid epidemic, prescribed opioids, and easy access drive this epidemic of overdoses, and opioid use disorders (OUDs). Presently the clinical consensus is to treat OUD, as if it were an opioid deficiency syndrome, with long-term to life-long opioid substitution therapy. Opioid agonist administration is seen as necessary to replace missing opioids, treat OUD, and prevent overdoses, like insulin is used to treat diabetes. Treatment of OUD and addiction, in general, is similar to the endocrinopathy conceptualization in that it views opioid agonist MATs as an essential core to therapy. Is this approach logical? Other than as harm reduction, is using opioids to treat OUD therapeutic or harmful in the long term? This historical Trieste provides a molecular framework to understand the current underpinnings of endorphinergic/dopaminergic mechanisms related to opioid deficiency syndrome and generalized reward processing depletion.
WC 249.
Highlights
-
•
Historical psychopharmacological work involved neurotransmission of serotonin, endorphins, glutamate, and dopamine, and the seminal work of Blum, Gold, Volkow, Nestler, and others related to neurotransmitter function and related behaviors.
-
•
The molecular framework to understand the current underpinnings of endorphinergic/dopaminergic mechanisms related to opioid deficiency syndrome and generalized reward processing depletion.
-
•
Presently the clinical consensus is to treat OUD, as if it were an opioid deficiency syndrome, with long-term to life-long opioid substitution therapy.
-
•
Opioid agonist administration is seen as necessary to replace missing opioids, treat OUD, and prevent overdoses, like insulin is used to treat diabetes. Is this approach logical?
Related collections
Most cited references166
- Record: found
- Abstract: found
- Article: not found
Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats.
- Record: found
- Abstract: found
- Article: not found
Epigenetics: definition, mechanisms and clinical perspective.
- Record: found
- Abstract: found
- Article: not found
