- Record: found
- Abstract: found
- Article: found
Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study
Read this article at
Abstract
Introduction
Vital drugs may be degraded or sequestered in extracorporeal membrane oxygenation (ECMO) circuits, with lipophilic drugs considered to be particularly vulnerable. However, the circuit effects on protein-bound drugs have not been fully elucidated. The aim of this experimental study was to investigate the influence of plasma protein binding on drug disposition in ex vivo ECMO circuits.
Methods
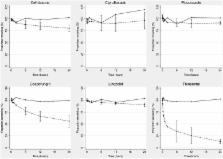
Four identical ECMO circuits comprising centrifugal pumps and polymethylpentene oxygenators and were used. The circuits were primed with crystalloid, albumin and fresh human whole blood and maintained at a physiological pH and temperature for 24 hours. After baseline sampling, known quantities of study drugs (ceftriaxone, ciprofloxacin, linezolid, fluconazole, caspofungin and thiopentone) were injected into the circuit to achieve therapeutic concentrations. Equivalent doses of these drugs were also injected into four polypropylene jars containing fresh human whole blood for drug stability testing. Serial blood samples were collected from the controls and the ECMO circuits over 24 hours, and the concentrations of the study drugs were quantified using validated chromatographic assays. A regression model was constructed to examine the relationship between circuit drug recovery as the dependent variable and protein binding and partition coefficient (a measure of lipophilicity) as explanatory variables.
Results
Four hundred eighty samples were analysed. There was no significant loss of any study drugs in the controls over 24 hours. The average drug recoveries from the ECMO circuits at 24 hours were as follows: ciprofloxacin 96%, linezolid 91%, fluconazole 91%, ceftriaxone 80%, caspofungin 56% and thiopentone 12%. There was a significant reduction of ceftriaxone ( P = 0.01), caspofungin ( P = 0.01) and thiopentone ( P = 0.008) concentrations in the ECMO circuit at 24 hours. Both protein binding and partition coefficient were highly significant, with the model possessing a high coefficient of determination ( R 2 = 0.88, P <0.001).
Conclusions
Recovery of the highly protein-bound drugs ceftriaxone, caspofungin and thiopentone was significantly lower in the ECMO circuits at 24 hours. For drugs with similar lipophilicity, the extent of protein binding may determine circuit drug loss. Future clinical population pharmacokinetic studies should initially be focused on drugs with greater lipophilicity and protein binding, and therapeutic drug monitoring should be strongly considered with the use of such drugs.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Pharmacokinetic issues for antibiotics in the critically ill patient.
- Record: found
- Abstract: found
- Article: not found
Extracorporeal membrane oxygenation in cardiopulmonary disease in adults.
- Record: found
- Abstract: found
- Article: not found