- Record: found
- Abstract: found
- Article: not found
G6b-B regulates an essential step in megakaryocyte maturation
Read this article at
Key Points
Abstract
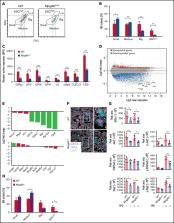
G6b-B is a megakaryocyte lineage-specific immunoreceptor tyrosine-based inhibition motif–containing receptor, essential for platelet homeostasis. Mice with a genomic deletion of the entire Mpig6b locus develop severe macrothrombocytopenia and myelofibrosis, which is reflected in humans with null mutations in MPIG6B. The current model proposes that megakaryocytes lacking G6b-B develop normally, whereas proplatelet release is hampered, but the underlying molecular mechanism remains unclear. We report on a spontaneous recessive single nucleotide mutation in C57BL/6 mice, localized within the intronic region of the Mpig6b locus that abolishes G6b-B expression and reproduces macrothrombocytopenia, myelofibrosis, and osteosclerosis. As the mutation is based on a single-nucleotide exchange, Mpig6b mut mice represent an ideal model to study the role of G6b-B. Megakaryocytes from these mice were smaller, displayed a less-developed demarcation membrane system, and had a reduced expression of receptors. RNA sequencing revealed a striking global reduction in the level of megakaryocyte-specific transcripts, in conjunction with decreased protein levels of the transcription factor GATA-1 and impaired thrombopoietin signaling. The reduced number of mature MKs in the bone marrow was corroborated on a newly developed Mpig6b-null mouse strain. Our findings highlight an unexpected essential role of G6b-B in the early differentiation within the megakaryocytic lineage.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: found
The incredible journey: From megakaryocyte development to platelet formation
- Record: found
- Abstract: found
- Article: not found
TNF-α–driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging
- Record: found
- Abstract: found
- Article: not found
