- Record: found
- Abstract: found
- Article: found
Tumor angiogenesis at baseline identified by 18F-Alfatide II PET/CT may predict survival among patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy
Read this article at
Abstract
Background
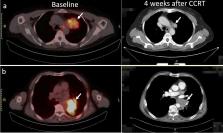
The study investigated the predictive value of tumor angiogenesis observed by 18F-ALF-NOTA-PRGD2 II (denoted as 18F-Alfatide II) positron emission tomography (PET)/computed tomography (CT) before concurrent chemoradiotherapy (CCRT) for treatment response and survival among patients with locally advanced non-small cell lung cancer (LA-NSCLC).
Methods
Patients with unresectable stage IIIA or IIIB NSCLC (AJCC Cancer Staging 7th Edition) who received CCRT were included in this prospective study. All patients had undergone 18F-Alfatide PET/CT scanning before CCRT, and analyzed parameters included maximum uptake values (SUV max) of primary tumor (SUV P) and metastatic lymph nodes (SUV LN) and mean uptake value of blood pool (SUV blood). Tumor-to-background ratios (TBRs) and changes in tumor diameter before and after CCRT (ΔD) were calculated. The ratios of SUV P to SUV blood, SUV LN to SUV blood, and SUV P to SUV LN were denoted as TBR P, TBR LN, and T/LN. Short-term treatment response, progression-free survival (PFS), and overall survival (OS) were evaluated.
Results
Of 38 enrolled patients, 28 completed CCRT. SUV P, SUV LN, TBR P, TBR LN and T/LN showed significant correlation with PFS (all P < 0.05). SUV P was negatively correlated with OS ( P = 0.005). SUV P and TBR P were higher in non-responders than in responders (6.55 ± 2.74 vs. 4.61 ± 1.94, P = 0.039; 10.49 ± 7.58 vs. 7.73 ± 6.09, P = 0.023). ΔD was significantly greater in responders (2.78 ± 1.37) than in non-responders (-0.16 ± 1.33, P < 0.001). Exploratory receiver operating characteristic curve analysis identified TBR P (area under the curve [AUC] = 0.764, P = 0.018), with a cutoff value of 6.52, as the only parameter significantly predictive of the response to CCRT, with sensitivity, specificity, and accuracy values of 71.43%, 78.57%, and 75.00%, respectively. ROC curve analysis also identified SUV P (AUC = 0.942, P < 0.001, cutoff value 4.64) and TBR P (AUC = 0.895, P = 0.001, cutoff value 4.95) as predictive of OS with high sensitivity (84.21%, 93.75%), specificity (100.00%, 66.67%), and accuracy (89.29%, 82.14%).
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy.
- Record: found
- Abstract: found
- Article: not found
Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer.
- Record: found
- Abstract: found
- Article: not found