- Record: found
- Abstract: found
- Article: found
Multienzyme Coimmobilization on Triheterofunctional Supports

Read this article at
Abstract
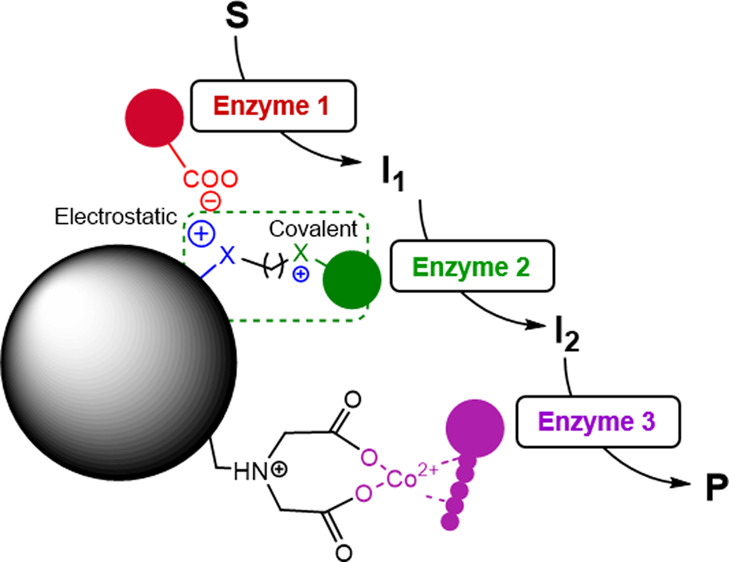
Immobilized multienzyme systems are gaining momentum in applied biocatalysis; however, the coimmobilization of several enzymes on one carrier is still challenging. In this work, we exploited a heterofunctional support activated with three different chemical functionalities to immobilize a wide variety of different enzymes. This support is based on agarose microbeads activated with aldehyde, amino, and cobalt chelate moieties that allow a fast and irreversible immobilization of enzymes, enhancing the thermostability of most of the heterogeneous biocatalysts (up to 21-fold higher than the soluble one). Furthermore, this trifunctional support serves to efficiently coimmobilize a multienzyme system composed of an alcohol dehydrogenase, a reduced nicotinamide adenine dinucleotide (NADH) oxidase, and a catalase. The confined multienzymatic system demonstrates higher performance than its free counterpart, achieving a total turnover number (TTN) of 1 × 10 5 during five batch consecutive cycles. We envision this solid material as a platform for coimmobilizing multienzyme systems with enhanced properties to catalyze stepwise biotransformations.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Fiji: an open-source platform for biological-image analysis.
- Record: found
- Abstract: found
- Article: not found
Modifying enzyme activity and selectivity by immobilization.
- Record: found
- Abstract: not found
- Article: not found