- Record: found
- Abstract: found
- Article: found
Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama
Read this article at
Abstract
Implanted biomaterials play a key role in current success of orthopedic and trauma surgery. However, implant-related infections remain among the leading reasons for failure with high economical and social associated costs. According to the current knowledge, probably the most critical pathogenic event in the development of implant-related infection is biofilm formation, which starts immediately after bacterial adhesion on an implant and effectively protects the microorganisms from the immune system and systemic antibiotics. A rationale, modern prevention of biomaterial-associated infections should then specifically focus on inhibition of both bacterial adhesion and biofilm formation. Nonetheless, currently available prophylactic measures, although partially effective in reducing surgical site infections, are not based on the pathogenesis of biofilm-related infections and unacceptable high rates of septic complications, especially in high-risk patients and procedures, are still reported.
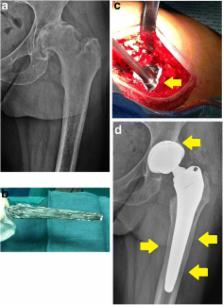
In the last decade, several studies have investigated the ability of implant surface modifications to minimize bacterial adhesion, inhibit biofilm formation, and provide effective bacterial killing to protect implanted biomaterials, even if there still is a great discrepancy between proposed and clinically implemented strategies and a lack of a common language to evaluate them.
To move a step forward towards a more systematic approach in this promising but complicated field, here we provide a detailed overview and an original classification of the various technologies under study or already in the market. We may distinguish the following: 1. Passive surface finishing/modification (PSM): passive coatings that do not release bactericidal agents to the surrounding tissues, but are aimed at preventing or reducing bacterial adhesion through surface chemistry and/or structure modifications; 2. Active surface finishing/modification (ASM): active coatings that feature pharmacologically active pre-incorporated bactericidal agents; and 3. Local carriers or coatings (LCC): local antibacterial carriers or coatings, biodegradable or not, applied at the time of the surgical procedure, immediately prior or at the same time of the implant and around it. Classifying different technologies may be useful in order to better compare different solutions, to improve the design of validation tests and, hopefully, to improve and speed up the regulatory process in this rapidly evolving field.
Related collections
Most cited references97
- Record: found
- Abstract: found
- Article: not found
Silver as antibacterial agent: ion, nanoparticle, and metal.
- Record: found
- Abstract: found
- Article: not found
Reducing implant-related infections: active release strategies.
- Record: found
- Abstract: found
- Article: not found